42 enthalpy diagram exothermic
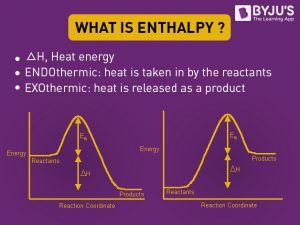
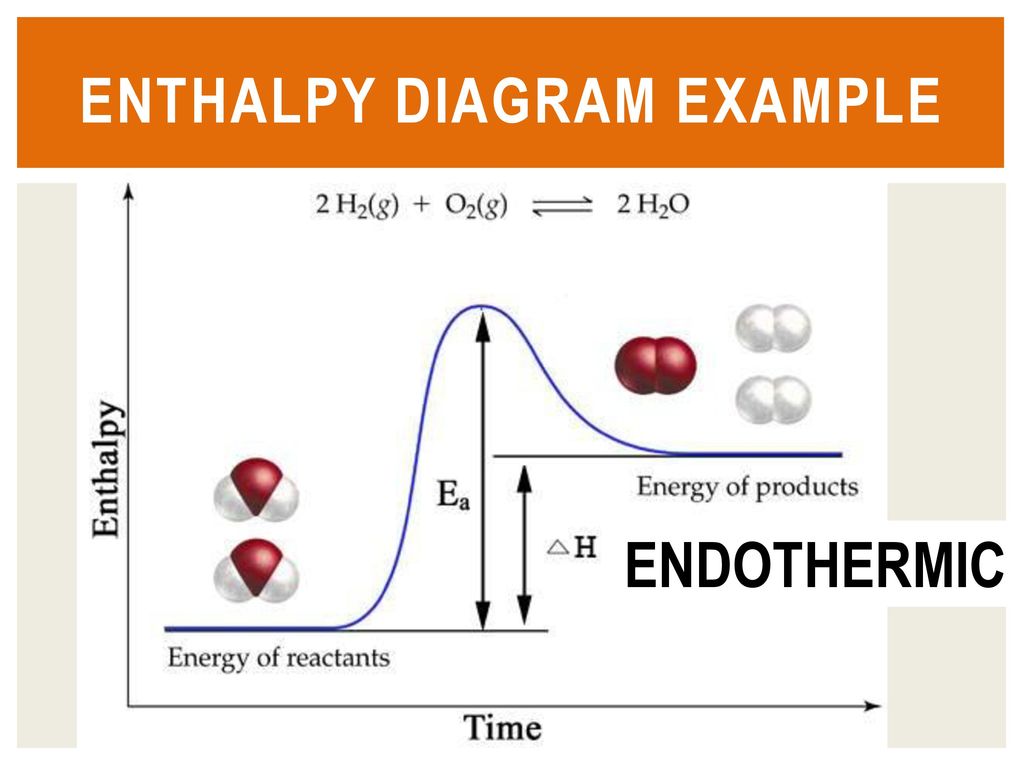
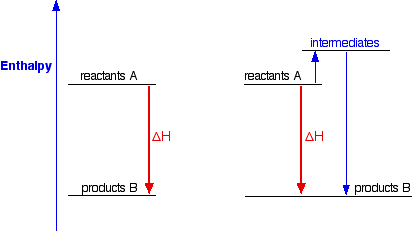
Draw the enthalpy diagram for exothermic and endothermic ... The enthalpy diagram for exothermic and endothermic reactions is shown below. Explanation : Endothermic reaction : It is defined as the chemical reaction in which the energy is absorbed from the surrounding. In the endothermic reaction, the energy of reactant are less than the energy of product. Endothermic vs. exothermic reactions (article) | Khan Academy The overall enthalpy of the reaction is negative, i.e., it's an exothermic reaction where energy is released in the form of heat. Depiction of an energy diagram In a chemical reaction, some bonds are broken and some bonds are formed.
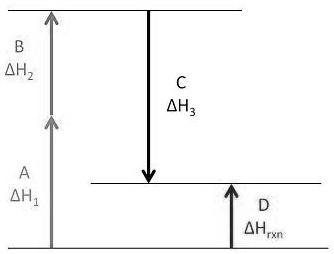
Enthalpy change of reactions | O Level Chemistry Notes We shall apply this formula to calculate the enthalpy change of combustion of hydrogen. STEP 1: Find the total bond energy of bonds broken Energy required to break 2 H-H = 2× 436 = 872 kJ/mol Energy required to break 1 O=O = 498 kJ/mol Total energy required for bond breaking = 872 + 498 = 1370 kJ/mol

Enthalpy diagram exothermic
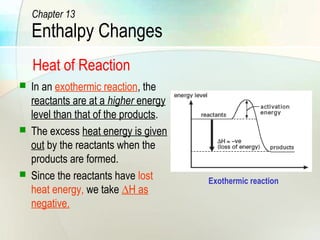
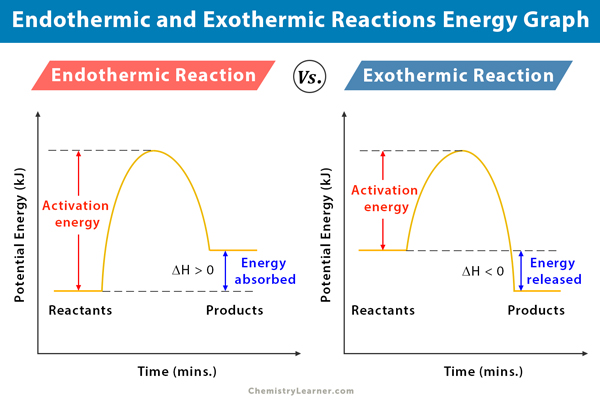
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2) Creative Diagram Of Exothermic Reaction - Glaucoma Template In an exothermic reaction change in enthalpy ΔH will be negative. A chemical reaction always involves a change in energy. An energy level diagram shows whether a reaction is exothermic or endothermic. Hesss law and reaction enthalpy change. In endothermic reactions the energy of the reactants is lower than that of the products. Energy From Chemicals - Enthalpy Change - Difference ... Energy Profile diagrams: Energy profile diagrams are used to represent the energy pathway from reactants to products in a reaction. The energy profile diagram for exothermic reaction show that the reactants have higher initial energy and products have lower energy since the energy has been given out during the reaction.
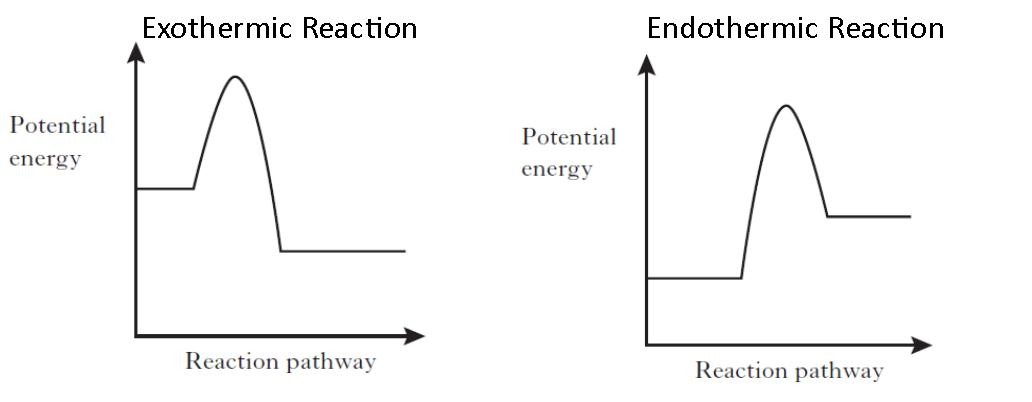
Enthalpy diagram exothermic. PDF Enthalpy Changes - WordPress.com Energy level diagram - exothermic These show the relative energy levels (enthalpy) of reactants and products: H 2+ Cl 2 (products) Enthalpy exothermic 2HCl (reactants) Note that the specific reactants and products are shown for the reaction taking place. Reaction profile diagram What is Enthalpy? - Definition, Endothermic & Exothermic ... Enthalpy change is the sum of internal energy denoted by U and product of volume and Pressure, denoted by PV, expressed in the following manner. H=U+PV. Enthalpy is also described as a state function completely based on state functions P, T and U. It is normally shown by the change in enthalpy (ΔH) of a process between the beginning and final states. How to Draw & Label Enthalpy Diagrams - Video & Lesson ... the molar enthalpy of formation of a compound is equal to the enthalpy change when one mole of the. Draw an energy diagram for the reaction: \\ CH_4(g) + 2O_2(g) \rightarrow CO_2(g) + 2H_2O(g ... PDF 5.1 - Exothermic and Endothermic Reactions Enthalpy level diagrams, or energy profile diagrams, allow us to visualise what happens to the enthalpy of a reaction as it proceeds The total enthalpy of the reactant species is labelled H R , the products H P The bonds between the reactants must first be broken before they can be converted into products.
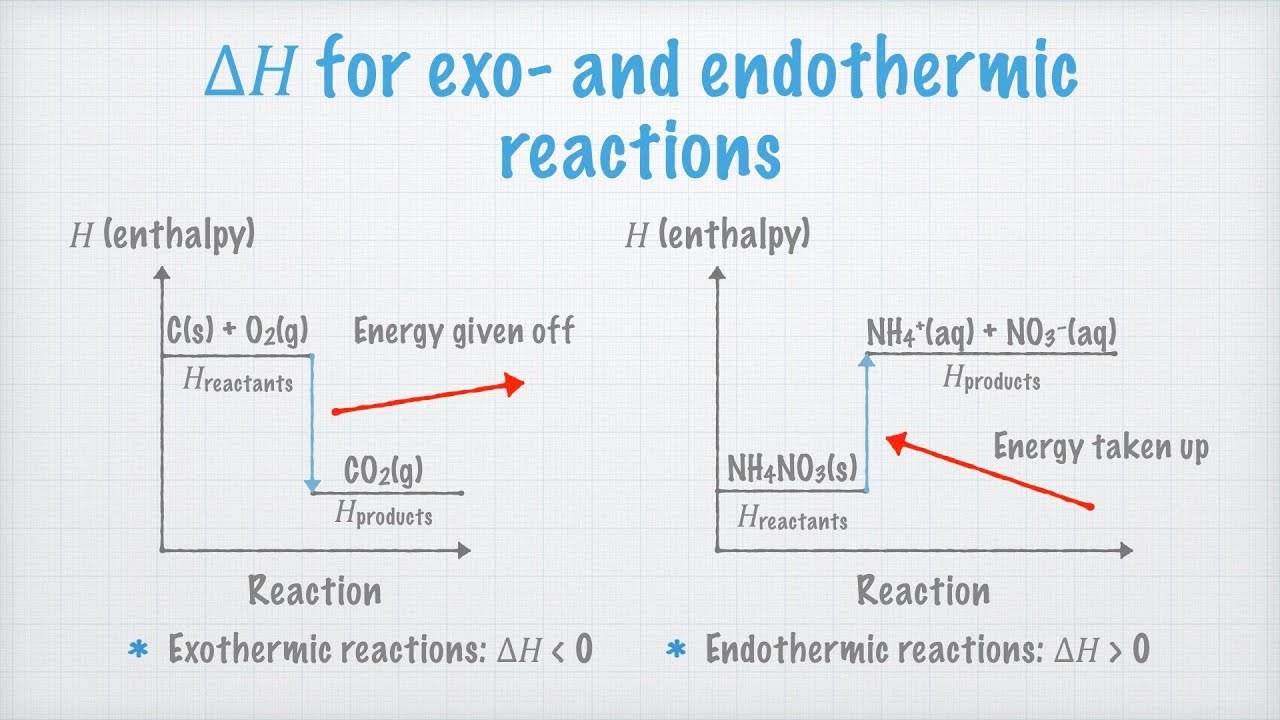
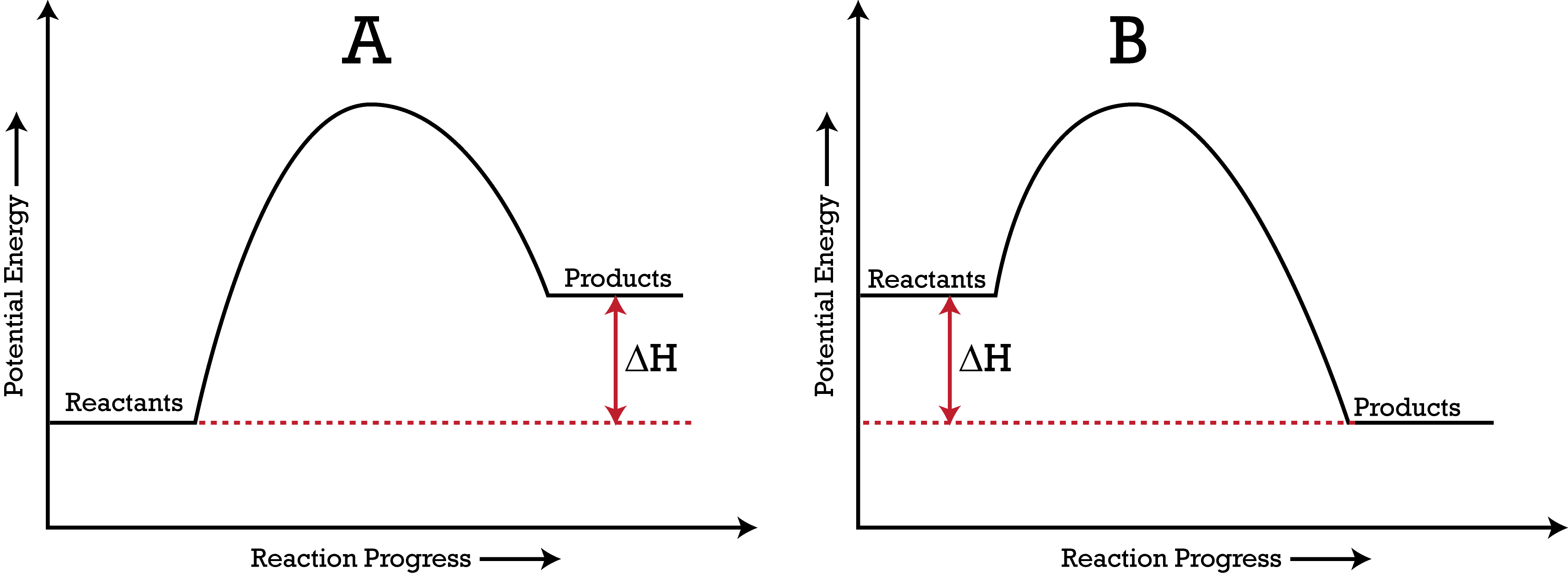
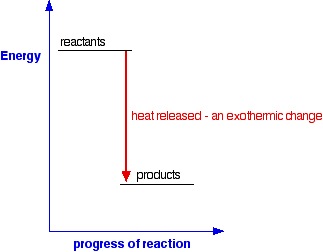
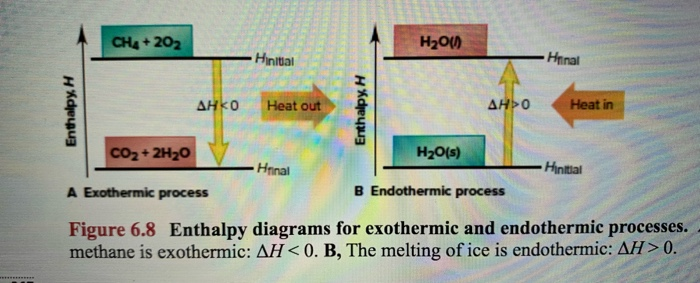
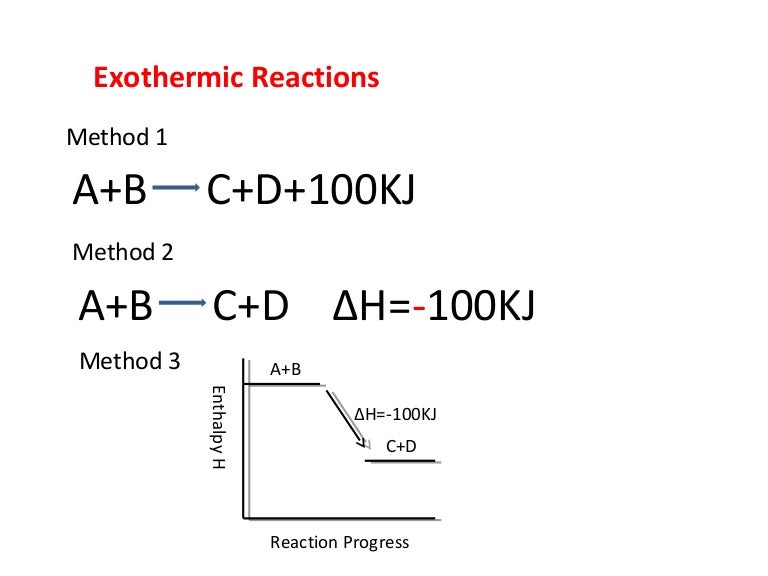
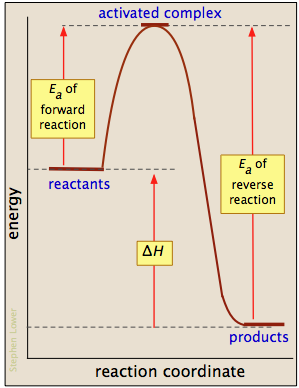
Enthalpy Change for Exothermic and ... - AUS-e-TUTE Enthalpy change for a chemical reaction (ΔH) is defined as the enthalpy of the products (H products) minus the enthalpy of the reactants (H reactants) ΔH = H (products) - H (reactants) An exothermic reaction is a chemical reaction or physical change in which heat is released. ⚛ Heat is a product of the reaction. Enthalpy - StudyPug The difference between exothermic and endothermic reactions is the amounts in those two steps: exothermic releases more, endothermic absorbs more. Energy in those diagrams above means chemical potential energy. This the combined attractive and repulsive forces in the atom or molecule. PDF ENTHALPY which take place in an exothermic chemical reaction. 2. The combustion of methane is an exothermic reaction: CH 4 + 2O 2 CO 2 + 2H 2 O ΔH = -890 kJmol-1 Draw an enthalpy profile diagram for the combustion of methane. Label the reactants and products, enthalpy change and activation energy. Explain why the enthalpy increases before it decreases. Energetics of a Reaction (6.1.1) | CIE ... - Save My Exams The enthalpy of the reactants and products is displayed on the y-axis The reaction pathway is shown on the x-axis Arrows on the diagrams indicate whether the reaction is exothermic ( downwards pointing) or endothermic ( upwards pointing) Diagrams showing the enthalpy change during an exothermic and endothermic reaction Exothermic:
Exothermic Energy Diagram: Activation Energy, Transition ... In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ... Energy Diagrams of Reactions | Fiveable Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. PDF Exothermic enthalpy diagram - Tangente Mag Gives or takes in heat energy, enthalpy change occurs.ΔH = Hproducts - HreactantsWhen petrol is burned enthalpy change is what really matters.ENTHALPY LEVEL DIAGRAMS:For Exothermic reaction ΔH is negative, it means the system loses energy to the surroundings. The products formed will have less energy than the reactants. Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In exothermic reactions, there is more energy in the reactants than ...
What is an enthalpy diagram? | Socratic An enthalpy diagram shows the change in enthalpy of the reaction as the chemicals move from reactants to products. The change in enthalpy is a fancy term for the change in thermal energy of the system at constant pressure. Typically we classify reactions as either endothermic or endothermic. Endothermic means the thermal energy of the products is more than that of the reactants. Exothermic ...
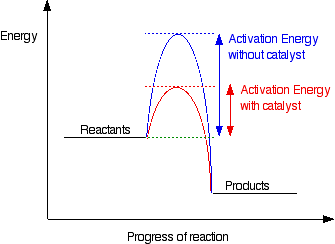
Exothermic Reaction - Definition and Examples | Properties ... Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings. In exothermic reactions, the reactants always possess more energy than the products and hence are less stable. For this reason, the exothermic reactions require very less amount of activation energy to initiate the reaction.
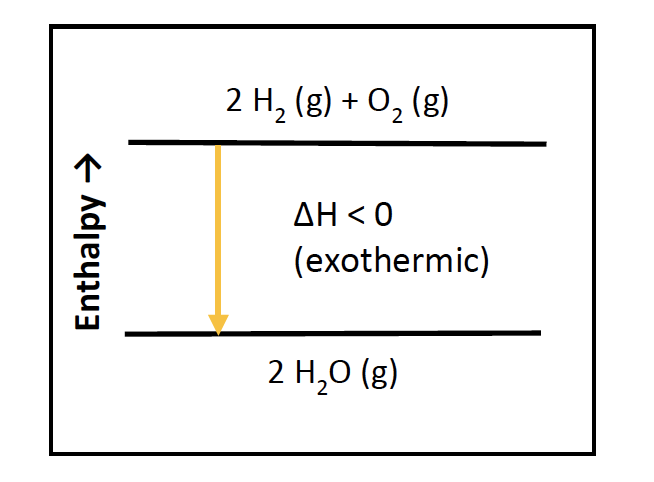
How does the energy level diagram show this reaction is ... (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. (c) The total energy of 1 mole of methane and 2 moles of oxygen is more than the total energy of 1 mole of carbon dioxide and 2 moles of water by 890 kJ.
SOLVED:Interpret Scientific Illustrations The reaction A ... Describe exothermic and endothermic reactions and factors that affect the ra… 01:13 Draw an enthalpy diagram for a general endothermic reaction; label the axis,…
Amazing Enthalpy Diagram For Exothermic Reaction Important ... I Indicate on your diagram the enthalpy change of the reaction and deduce its sign. An energy level diagram shows whether a reaction is exothermic or endothermic. Exothermic reaction in an exothermic reaction the total energy of the products is less than the total energy of the reactants. Step-by-step solution 100 5 ratings for this solution.
Exothermic and Endothermic Processes | Introduction to ... Enthalpy. Enthalpy (signified as H) is a measure of the total energy of a system and often expresses and simplifies energy transfer between systems. Since the total enthalpy of a system cannot be measured directly, we most often refer to the change in enthalpy for a particular chemical reaction. At constant pressure, the change in enthalpy is equal to the heat given off, or the heat absorbed, in a given chemical reaction:
AS 3.2.1 - Enthalpy profile diagrams explained / A level ... Hi everyone and welcome back to ASFC Chemistry!Click the little i to the top right hand corner of this video to be taken to some of our other A-level chemist...
Enthalpy | Boundless Chemistry - Lumen Learning Exothermic processes release energy upon completion, and are signified by a negative change in enthalpy. Key Terms. exothermic: Of a chemical reaction that releases energy in the form of heat. enthalpy: In thermodynamics, a measure of the heat content of a chemical or physical system. endothermic: Of a chemical reaction that absorbs heat energy ...
What are Exothermic Reactions? (with Examples and Video) The standard enthalpy change (ΔH) for the reaction between a proton (H+ ion) and a hydroxide ion (OH- ion) is -57.30 kJ/mol. Therefore, neutralization reactions are considered to be exothermic reactions. Why is Respiration an Exothermic Reaction? Respiration is the process by which humans take in oxygen and give out carbon dioxide.
Energy From Chemicals - Enthalpy Change - Difference ... Energy Profile diagrams: Energy profile diagrams are used to represent the energy pathway from reactants to products in a reaction. The energy profile diagram for exothermic reaction show that the reactants have higher initial energy and products have lower energy since the energy has been given out during the reaction.
Creative Diagram Of Exothermic Reaction - Glaucoma Template In an exothermic reaction change in enthalpy ΔH will be negative. A chemical reaction always involves a change in energy. An energy level diagram shows whether a reaction is exothermic or endothermic. Hesss law and reaction enthalpy change. In endothermic reactions the energy of the reactants is lower than that of the products.
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2)























Comments
Post a Comment