40 n2 molecular orbital diagram
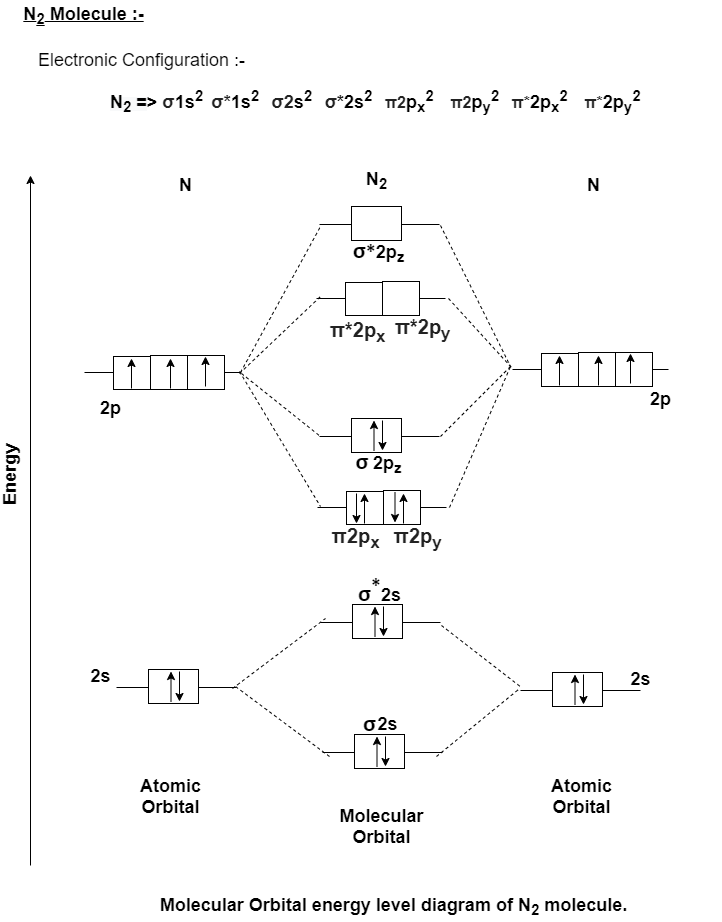
How is the molecular orbital diagram of N2 determined? - Quora Answer (1 of 2): Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bond orbital next 2 in 2s sigma... (Get Answer) - The molecular orbital diagram of N2 is ... The molecular orbital diagram of N2 is shown below. a) Fill in the electrons in the molecular orbital diagram for N2 b) Calculate the bond order of N2 Ouole bon d 2p c) Is N2 a stable compound?
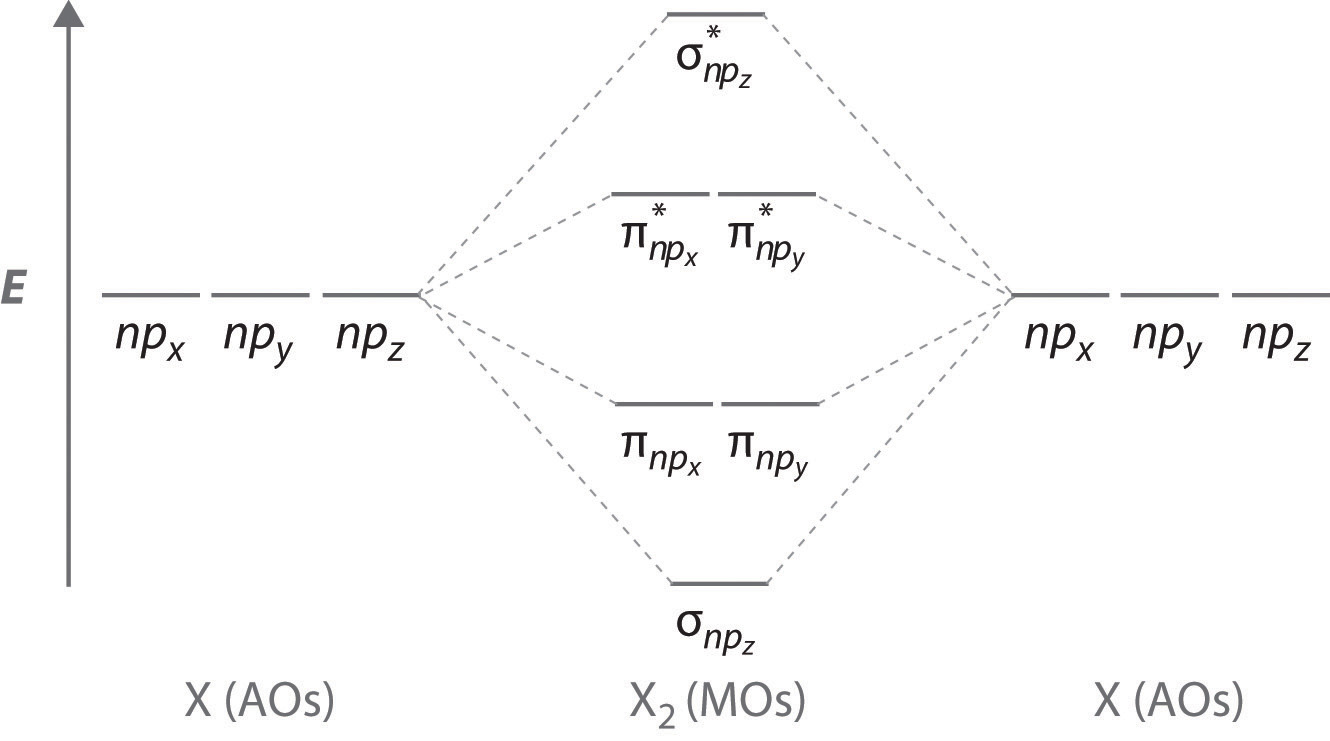
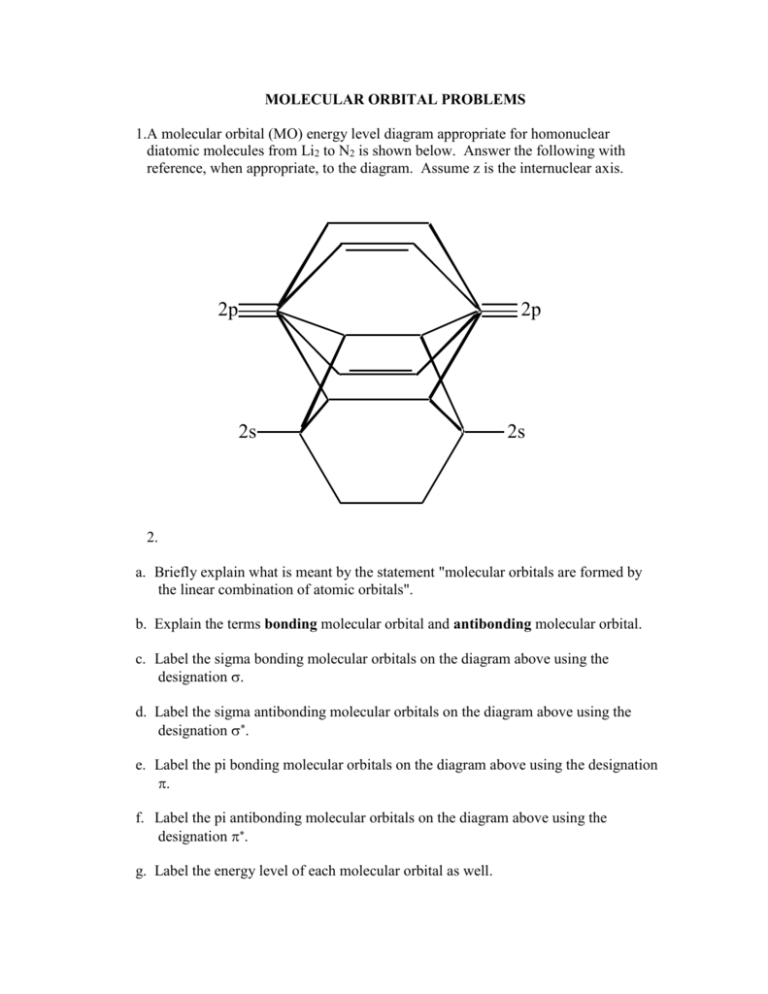
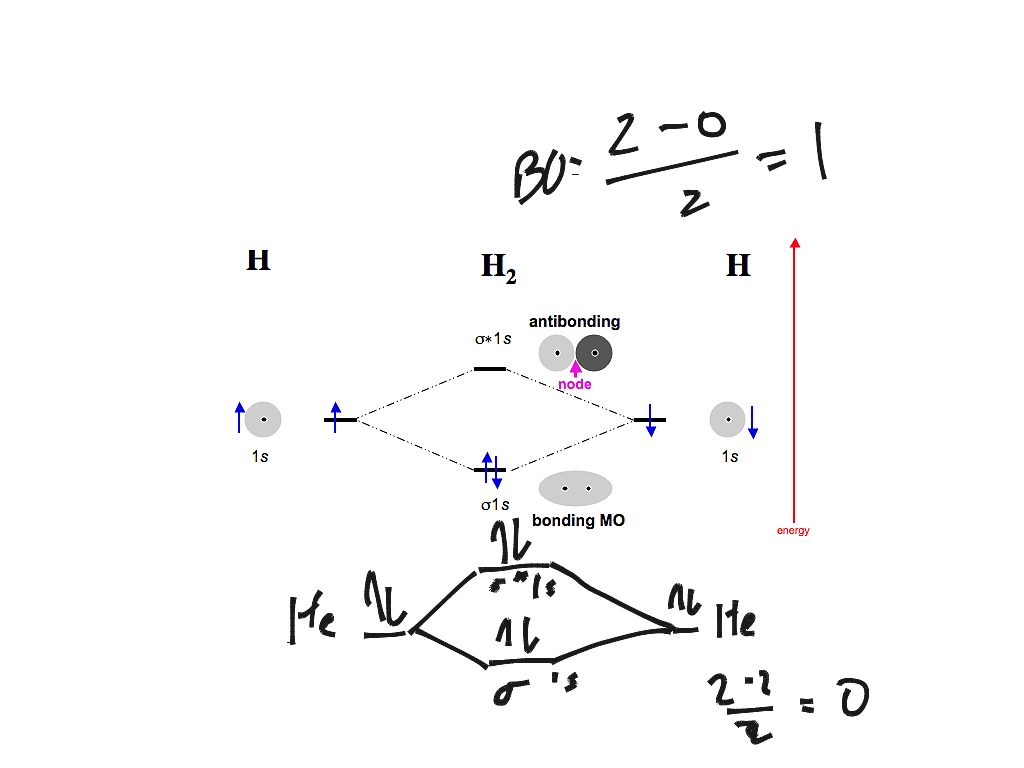
7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.
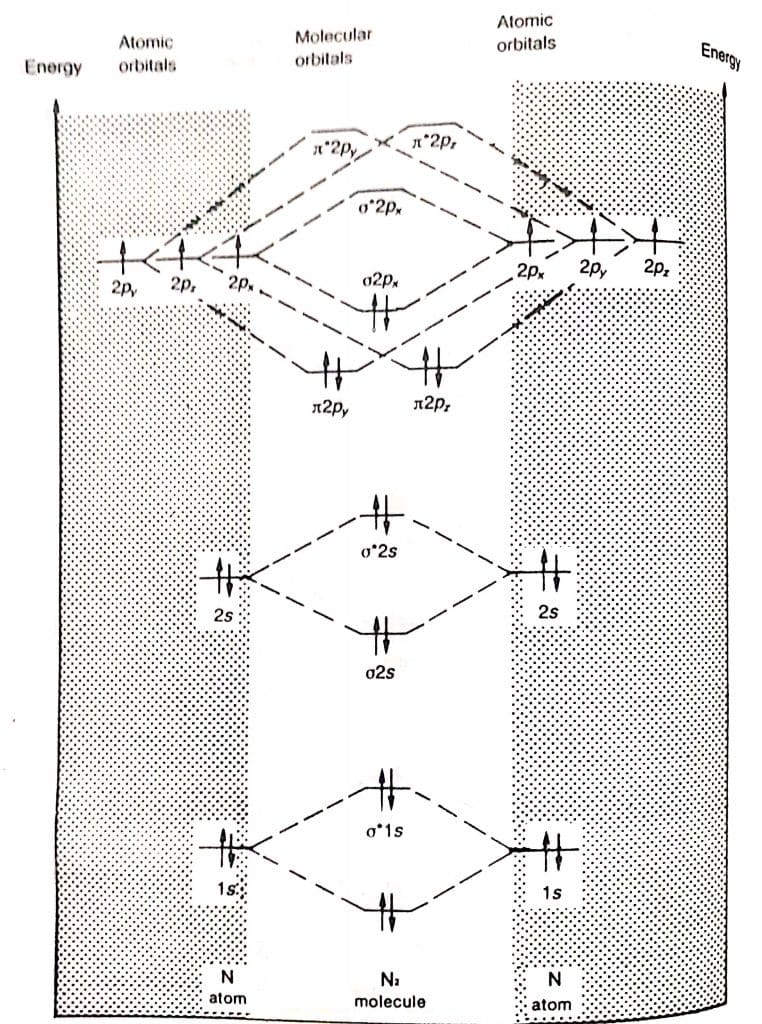
N2 molecular orbital diagram
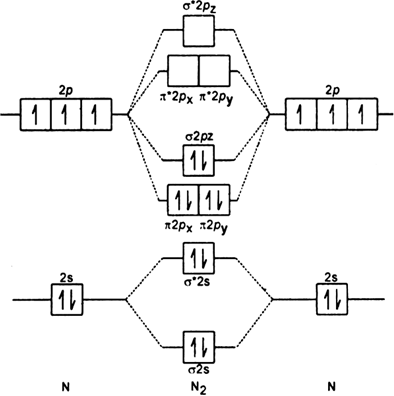
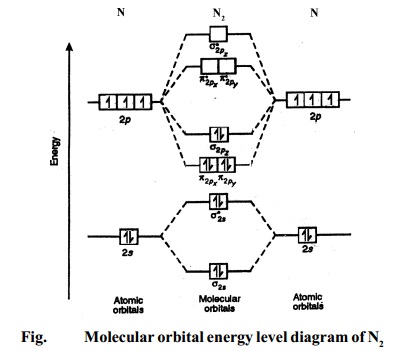
Draw molecular orbital diagram of O2 or N2 with magnetic ... Solution. , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b. ]/2=[10−6]/2=2. Therefore there is a double bond present as O=O. N2+ Mo Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram. Molecular orbitals in Nitrogen - ChemTube3D There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two perpendicular pi bonds.
N2 molecular orbital diagram. 42 molecular orbital diagram for n2 - Modern Wiring Diagram Molecular orbital diagram for n2. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. Molecular Nitrogen and Related Diatomic Molecules Here is the full molecular orbital diagram for N2. Now we add the 10 electrons, 5 from each nitrogen atom. Note that the bottom sigma symmetry orbital is strongly bonding, the top one is strongly antibonding, and the 2 in the middle are only weakly bonding and antibonding, respectively. PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in N2 Lewis Structure, Molecular Geometry, and Hybridization ... For more detailed knowledge you can refer to the polarity of N2. Molecular Orbital Diagram of N2 Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.
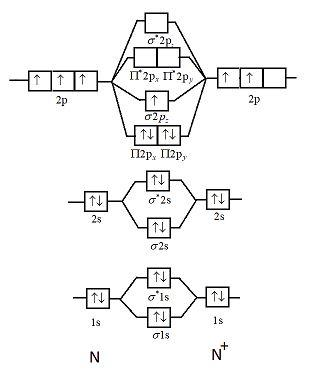
Draw the molecular orbital diagram of N2N2 + N2 Write ... This picture shows the molecular orbital diagram of N 2. Orbitals represented by ∗ are antibonding orbitals and the orbitals without ∗ are bonding orbitals. Bond order can be calculated by the formula: Bond order = bonding electrons - antibonding electrons 2 N2 Lewis Structure: Full Guide (2022 Updated) N2 Molecular Orbital Diagram. Each molecule has its electron configuration consisting of a sigma bond and a pi bond, known as molecular orbitals. The molecular orbital theory determines the stability order, magnetic nature, and the number of bonds in a molecule. write the molecular orbital diagram of n2 and calculate ... The total number of electrons present in the N 2 molecule is 14. N 2+ ion is formed by the loss of one electron from the N 2 molecule. This lost electron will be lost from σ (2p z) orbital. Hence, the electronic configuration of N 2+ ion will be N 2+ = KK [σ (2s)] 2 [σ* (2s)] 2 [π (2p x )] 2 [π (2p y )] 2 [σ (2p z )] 1 Here, N b =7, N a =2 so that Draw molecular orbital diagram for N_(2)^(+) molecule. Drawn the molecular orbital diagram and write the bond order, magnetic properties of `N_(2)` molecule and `N_(2)^(o+)` ion ? Draw molecular orbital energy level diagram for nitrogen molecule. 417326630
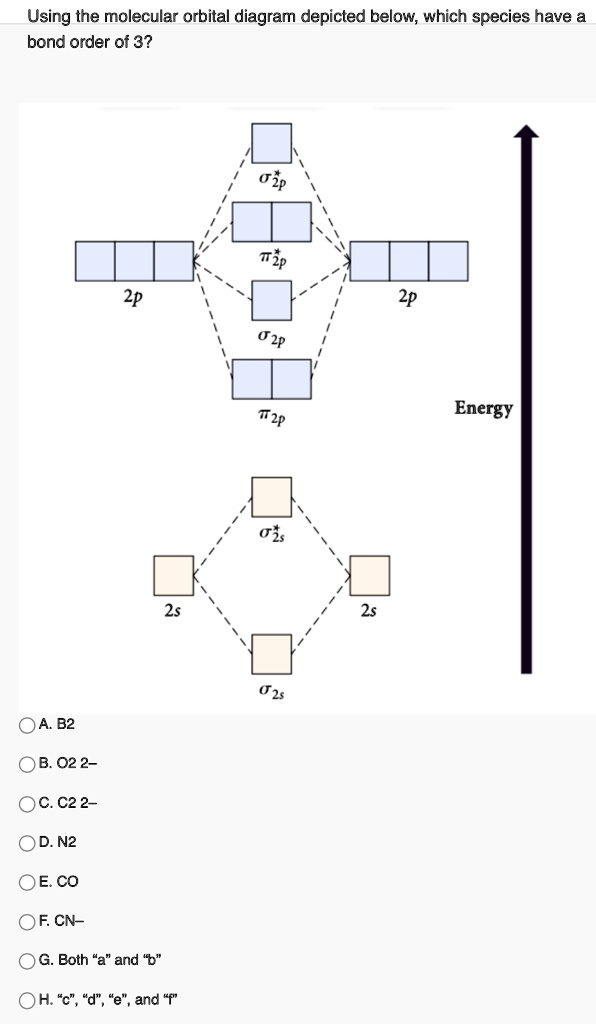
Draw the molecular orbital diagram of N2 and calculate the ... Draw the molecular orbital diagram of N2 and calculate the bond order. Answer: The bond order shows the number of chemical bonds present between a pair of atoms. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Bond order formula is given as below. Answered: A 30. Use the molecular orbital diagram… | bartleby A 30. Use the molecular orbital diagram to figure out the electr configuration for N2. The bond orders in N2', and N2 are A. 2.5 and 2.5 B. 2.5 and 2 C. 2.5 and 1.5 D. 1.5 and 2.5 E. 2 and 2.5 D 31. MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start... Molecular Orbitals for N2 - Newcastle University Molecular Orbitals for N2. Jmol models of calculated wavefunctions. To view a model, click on a molecular orbital in the energy level correlation diagram shown Mouse Control of Models. Left mouse drag to rotate; Shift Left drag up or down to resize; Shift Right drag or Shift Left drag horizontally to z-rotate; Right click for menu Notes Usage
Molecular Orbital Diagram For Ne2 We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory.
Solved 5. Draw the molecular orbital diagram for N2. Label ... Draw the molecular orbital diagram for N2. Label all of the atomic orbitals and molecular orbitals and put the correct number of electrons in. You do not need to draw the shapes of any of the orbitals. a) MO diagram b) Based on your MO diagram, is N2 diamagnetic or paramagnetic? c) Calculate the bond order for N2.
What are the molecular orbital configurations for N_2^+, N ... Organic Chemistry Hybridization and Atomic and Molecular Orbitals Molecular Orbitals and Hybridizations. 1 Answer Truong-Son N. Nov 2, 2015 If we build the MO diagram for #"N"_2#, it looks like this: First though, notice that the #p# orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be.
N2+ Mo Diagram - schematron.org the diagram above is the molecular.n2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level structures can construct the molecular orbital energy level the …
Draw a molecular orbital diagram of N2 or O2 with magnetic ... Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means.
draw the molecular orbital diagram of n2 also find its ... Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric. Explain What is the relationship between bond order and the dissociation energy of a molecule? ...
Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
Molecular orbital (MO) diagram for N2 and N2^- Despite $\ce{N2-}$ being isoelectronic to $\ce{O2+}$, the lower effective nuclear charge on nitrogen should make its s orbitals a little closer to the energies of the p orbitals than they would be in oxygen. Hence s-p mixing should occur. ... Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion ...
Molecular orbitals in Nitrogen - ChemTube3D There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two perpendicular pi bonds.
N2+ Mo Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram.
Draw molecular orbital diagram of O2 or N2 with magnetic ... Solution. , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b. ]/2=[10−6]/2=2. Therefore there is a double bond present as O=O.

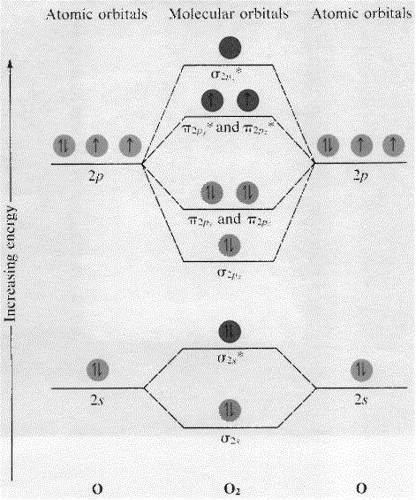


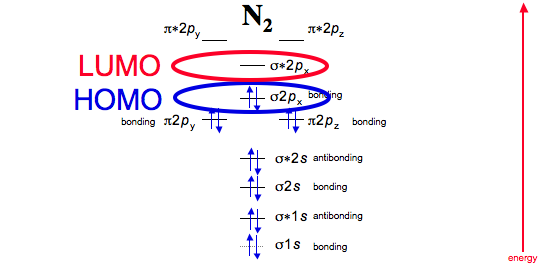
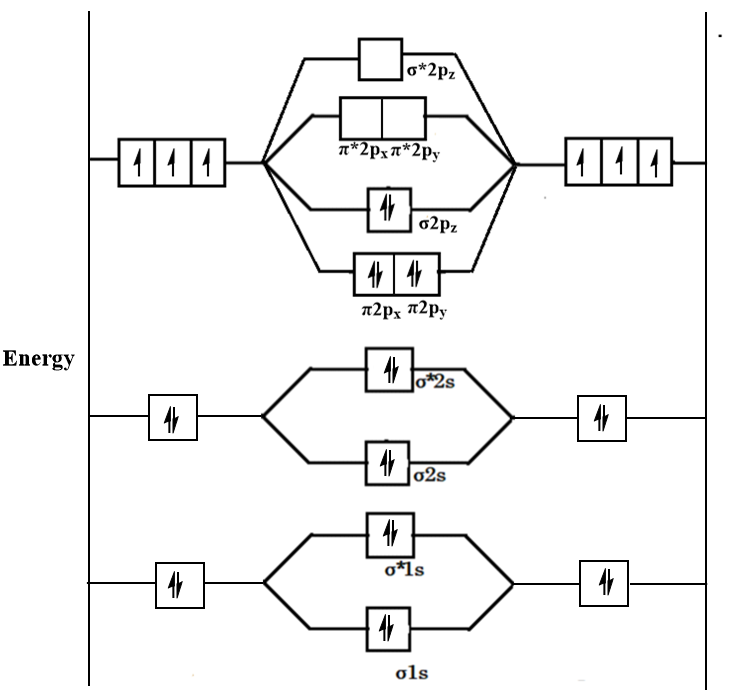

![Best Answer] draw the molecular orbital diagram of N2 and ...](https://hi-static.z-dn.net/files/d20/b492acf8cb9ff01954c3929a3b7a93c7.jpg)













Comments
Post a Comment