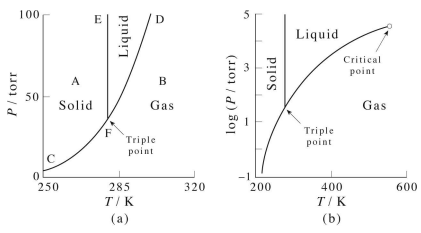
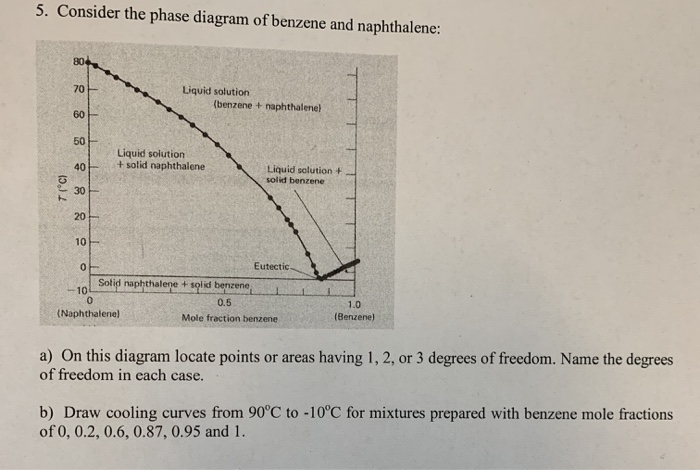
42 phase diagram of benzene
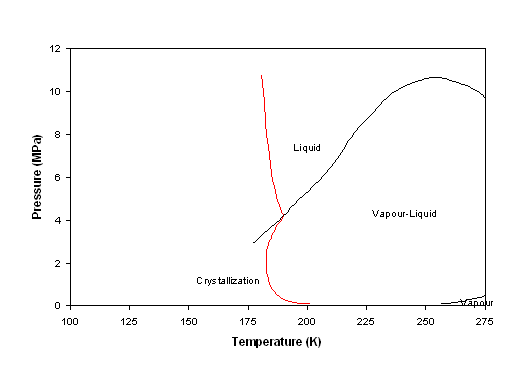
Titan is one other place in the solar system that has large amounts of these compounds. On Titan, organic molecules are produced in the atmosphere and carried to the surface where they can mineralize. Here, we report the phase diagram of mixtures of acetonitrile and benzene, and provide an account of the structure and composition of the phases. The phase diagram of benzene as it results from the present work. The full lines, identifying the melting line and the I-II phase boundary, and the two triple points (empty circles) are from Ref. 22. Dashed lines delimit the hypothesized phase IV, the low-temperature region of phase I, and the stability range of benzene (Ref. 15).
Nevertheless, the com- plete phase diagram is far from being completely un- derstood. At atmospheric pressure, benzene freezes at 278.5 K [ 19] into an orthorhombic D {~ phase with four molecules per unit cell [ 16]. The same phase is also formed at room temperature upon compres- sion above 0.07 GPa [ 19,22].

Phase diagram of benzene
The phase diagram of benzene has been determined to 35 kbar. Two triple points, liquid-benzene I-benzene II and liquid-benzene II-benzene III, have been located. These triple points are at 12 ± 0.5 kbar and 204 ± 5°C, and 22.5 ± 0.5 kbar and 335 ± 5°C, respectively. The benzene I-benzene II phase boundary is slightly convex towards the temperature axis. point we have pure benzene (1 mole fraction benzene), so the boiling point of benzene at 1 atm can be read from the diagram (353.0 K). In the region between the curves, there are two phases; in the region above the saturated vapor curve, there is only a single "superheated" vapor phase; in the region below the saturated liquid curve, there processes (such as phase equilibria). Phase Diagrams of Benzene. Phase transitions as function of P PE-2 1 atm. 2 atm. 3 atm. Benzene. At 1 atm the melting pt is 5.5 °C. At 34 atm the melting point is 6.5 °C. At 1 atm the boiling pt is 80.1 °C. At 0.66 atm the boiling point is 67 °C. MP at 1 atm = Normal .
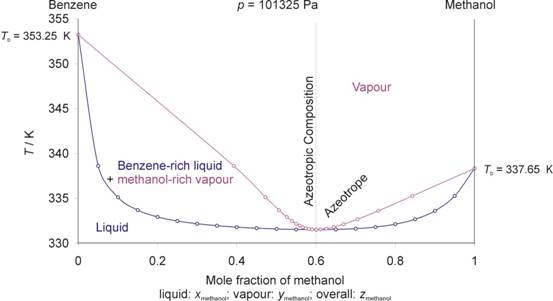
Phase diagram of benzene. The qualitative representation of the benzene phase diagram. Assuming experimental detection of the benzene metastability endpoint of the change in the thermodynamic parameters indicated in the work on a small interval is an example of a fast but still continuous phase transition (or, according to P. Ehrenfest's classification, a phase ... is only a single "subcooled" liquid phase. Figure 1.2 Txy diagram for benzene and toluene at 1atm. BINARY VLE PHASE DIAGRAMS 3. The diagram is easily generated in Aspen Plus by going to Tools on the upper tool bar and selecting Analysis, Property, and Binary. The window shown in Figure 1.3 opens on Benzene is present in crude oils and is a product of oil-refining processes. There are limitations on the content of benzene in gasoline. In industry benzene is used as a solvent, as a chemical intermediate, and is used in the synthesis of numerous chemicals. The phase diagram of benzene is shown below the table. A temperature versus pore diameter phase diagram of confined benzene is proposed combining liquid, supercooled liquid, crystal states, and glassy states. NMR relaxation time measurements showed that the dynamics of the confined liquids are slower than those of the bulk above its melting point.
For benzene (C 6 H 6), the normal boiling point is 80.1 °C and the enthalpy of vaporization is 30.8 kJ/mol. What is the boiling point of benzene in Denver, where atmospheric pressure = 83.4 kPa? ... Phase diagrams can be used to determine and predict the phase of a substance at a given temperature and pressure and also the phase changes that ... Phase Diagrams: Problem 10.82: Look at the phase diagram of CO 2 in Figure 10.29 and tell what phases are present under the following conditions: Before answering the questions below, we first bring up the phase diagram. Now we are ready to answer the questions. Just look at the graph and compare to the answers given below. As emerges from Figure 13.1, Raoult's law divides the diagram into two distinct areas, each with three degrees of freedom. 57 Each area contains a phase, with the vapor at the bottom (low pressure), and the liquid at the top (high pressure). Raoult's law acts as an additional constraint for the points sitting on the line. Therefore, the number of independent variables along the line is ... A new method to determine a ternary phase diagram of benzene-acetic acid-water system is provided to upper-division undergraduate students. The partially miscible liquid phase region can be accurately constructed using isothermal titration microcalorimetry (ITC). Compared with the traditional visual method, ITC can provide precise data and more information about the dissolution process despite ...
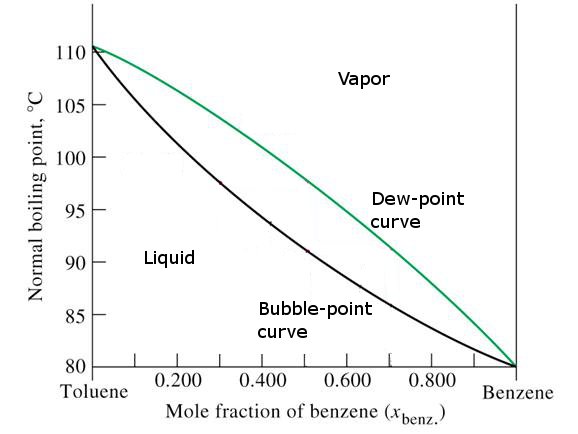
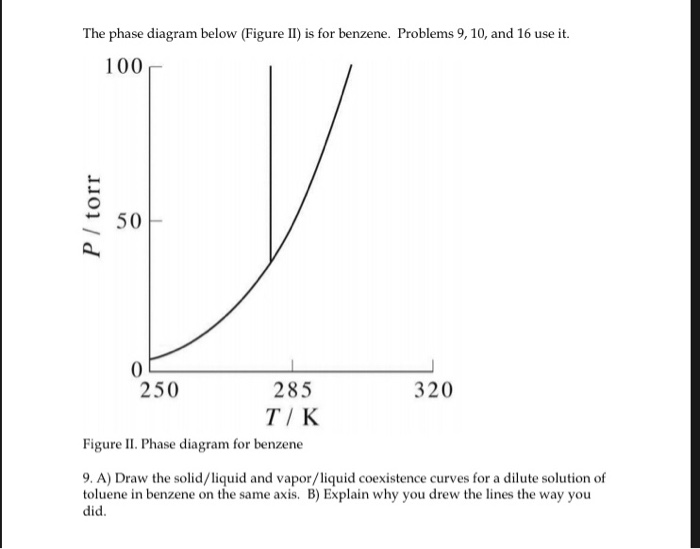
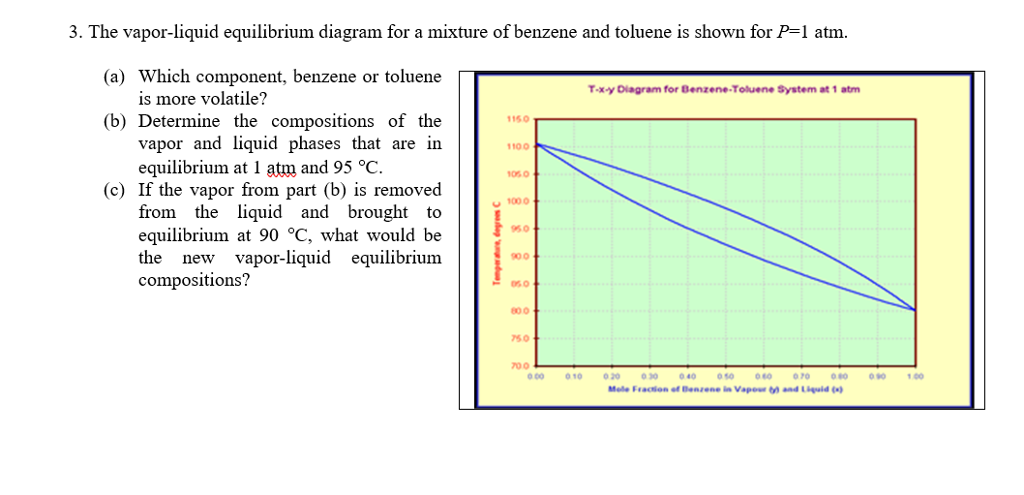
Sketch the phase diagram of benzene.Make sure to label the axes and the different phases of benzene.Use the physical data provided below. melting point = 279 K boiling point = 353 K Tc = 562 K Pc = 48.4 atm Triple Point = 0.05 atm, 279 K Dec 09, 2003 · The phase diagram of benzene has been determined to 35 kbar. Two triple points, liquid‐benzene I–benzene II and liquid‐benzene II–benzene III, have been located. These triple points are at 12 ± 0.5 kbar and 204 ± 5°C, and 22.5 ± 0.5 kbar and 335 ± 5°C, respectively. Figure 1.2 gives the Txy diagram for the benzene/toluene system at a pressure of 1 atm. The abscissa shows the mole fraction of benzene; the ordinate, temperature. The lower curve is the "saturated liquid" line, which gives the mole fraction of benzene in the liquid phase x. Answer (1 of 2): Benzene can exist as a two-phase liquid and gas mixture over a range of temperatures depending on the pressure. At 1 atm pressure that is 80.1 deg C. (This discussion is analogous to steam-water two phase mixtures and the phase diagram for water. It may be challenging to find th...
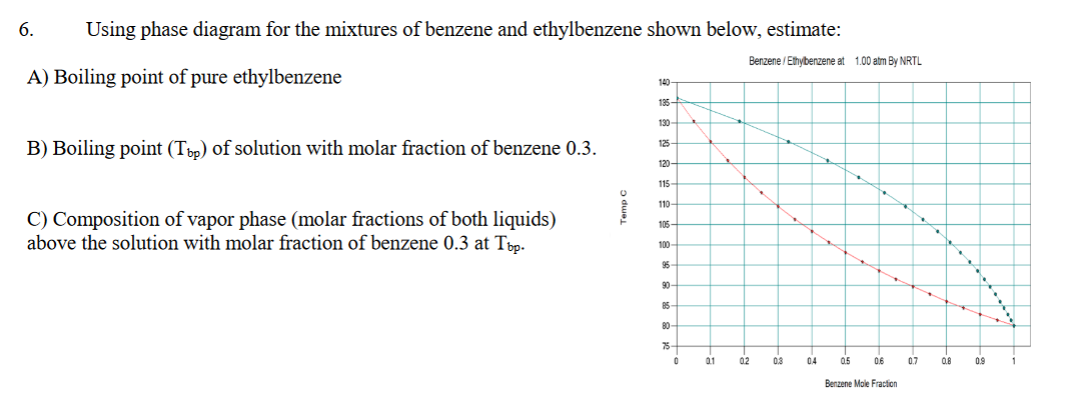
diagrams of the systems benzene-toluene and benzene-ethylbenzene. Kramer (1) and Richiardi (2) have investigated the equilibrium diagrams of other binary systems of benzene and mono-substituted benzenes. The information contained in their papers is the only data available related to this problem. Methods available . tor
Embed figure. Phase diagram for water-benzene mixtures. The thick solid lines: one-phase critical curve and liquid-liquid-gas three phase curve; c.p is the critical points and the diamond symbols ...
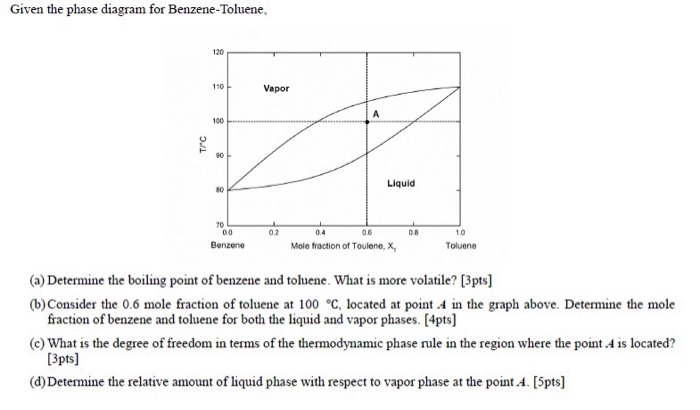
In the diagram for a benzene-toluene mixture at 20˚C shown, the solid blue line indicates the composition of the liquid and the solid green line indicates the composition of the vapor. For a point inside the two-phase region, the compositions of the two phases are determined by drawing horizontal lines (here shown dashed).
Aug 25, 2020 · File:Benzene phase diagram.svg. Size of this PNG preview of this SVG file: 360 × 330 pixels. Other resolutions: 262 × 240 pixels | 524 × 480 pixels | 838 × 768 pixels | 1,117 × 1,024 pixels | 2,234 × 2,048 pixels.
Benzene | C6H6 - PubChem. National Center for Biotechnology Information. 8600 Rockville Pike, Bethesda, MD, 20894 USA. Contact. Policies. FOIA. National Library of Medicine. National Institutes of Health. Department of Health and Human Services.
Construct a Txy diagram for a mixture of benzene and toluene at 200 kPa. Benzene and toluene mixtures may be considered as ideal. Data: Vapor pressure, P sat, data: ln P = A − B/( T + C), where P is in kPa and T is in K. Compound A B C Benzene (1) 14.1603 2948.78 − 44.5633
High-pressure and high-temperature equation of state and phase diagram of solid benzene. The high-pressure structural properties of benzene have been investigated up to the occurrence of the chemical reaction by means of x-ray diffraction and infrared absorption techniques. The infrared spectra show that sample annealing above 500 K produces ...
Typical liquid-phase reaction conditions for the chlorination of benzene using FeCl3 catalyst are 80-100°C and atmospheric pressure. When a high benzene/Cl2 ratio is used, the product mixture is approximately 80% monochlorobenzene, 15% p-dichlorobenzene and 5% o-dichlorobenzene.
A vapor pressure diagram shows the regions of phase stability for a two-component mixture as a function of pressure and composition at a fixed temperature. Since benzene and toluene form a nearly ideal solution, the vapor pressure diagram for this mixture can be calculated from the vapor pressures of the two pure substances at a given temperature.
Temperature (K) A B C Reference Comment; 333.4 - 373.5: 4.72583: 1660.652-1.461: Eon, Pommier, et al., 1971: Coefficents calculated by NIST from author's data.
Jan 20, 2022 · Benzene Phase Diagram. Here are a number of highest rated Benzene Phase Diagram pictures upon internet. We identified it from honorable source. Its submitted by management in the best field. We agree to this kind of Benzene Phase Diagram graphic could possibly be the most trending subject gone we allocation it in google pro or facebook.
In the first part of the work, thephase diagram of the benzene -ndash;[CuPy4(NO3)2] system has beendetermined in the -100 to +200 °C temperaturerange using DTA and solubility techniques. The onlycompound found in the system is the[CuPy4(NO3)2]β 2C6H6clathrate. It is stable up to a temperature of+104.2(5) °C at which it melts incongruently togive liquid and the solid [CuPy4(NO3)2]host phase ...
processes (such as phase equilibria). Phase Diagrams of Benzene. Phase transitions as function of P PE-2 1 atm. 2 atm. 3 atm. Benzene. At 1 atm the melting pt is 5.5 °C. At 34 atm the melting point is 6.5 °C. At 1 atm the boiling pt is 80.1 °C. At 0.66 atm the boiling point is 67 °C. MP at 1 atm = Normal .
point we have pure benzene (1 mole fraction benzene), so the boiling point of benzene at 1 atm can be read from the diagram (353.0 K). In the region between the curves, there are two phases; in the region above the saturated vapor curve, there is only a single "superheated" vapor phase; in the region below the saturated liquid curve, there
The phase diagram of benzene has been determined to 35 kbar. Two triple points, liquid-benzene I-benzene II and liquid-benzene II-benzene III, have been located. These triple points are at 12 ± 0.5 kbar and 204 ± 5°C, and 22.5 ± 0.5 kbar and 335 ± 5°C, respectively. The benzene I-benzene II phase boundary is slightly convex towards the temperature axis.















![T-P phase diagram [1] for the fluid-phase I of benzene ...](https://www.researchgate.net/profile/Kamil_Savas/publication/283676635/figure/download/fig1/AS:654417502158864@1533036699823/T-P-phase-diagram-1-for-the-fluid-phase-I-of-benzene.png)
![( a ) Phase diagram of benzene [8]. Blue dots indicate the ...](https://www.researchgate.net/profile/Margherita-Citroni/publication/262113326/figure/download/fig1/AS:296824918298626@1447779984001/a-Phase-diagram-of-benzene-8-Blue-dots-indicate-the-experimental-threshold-point.png)






Comments
Post a Comment