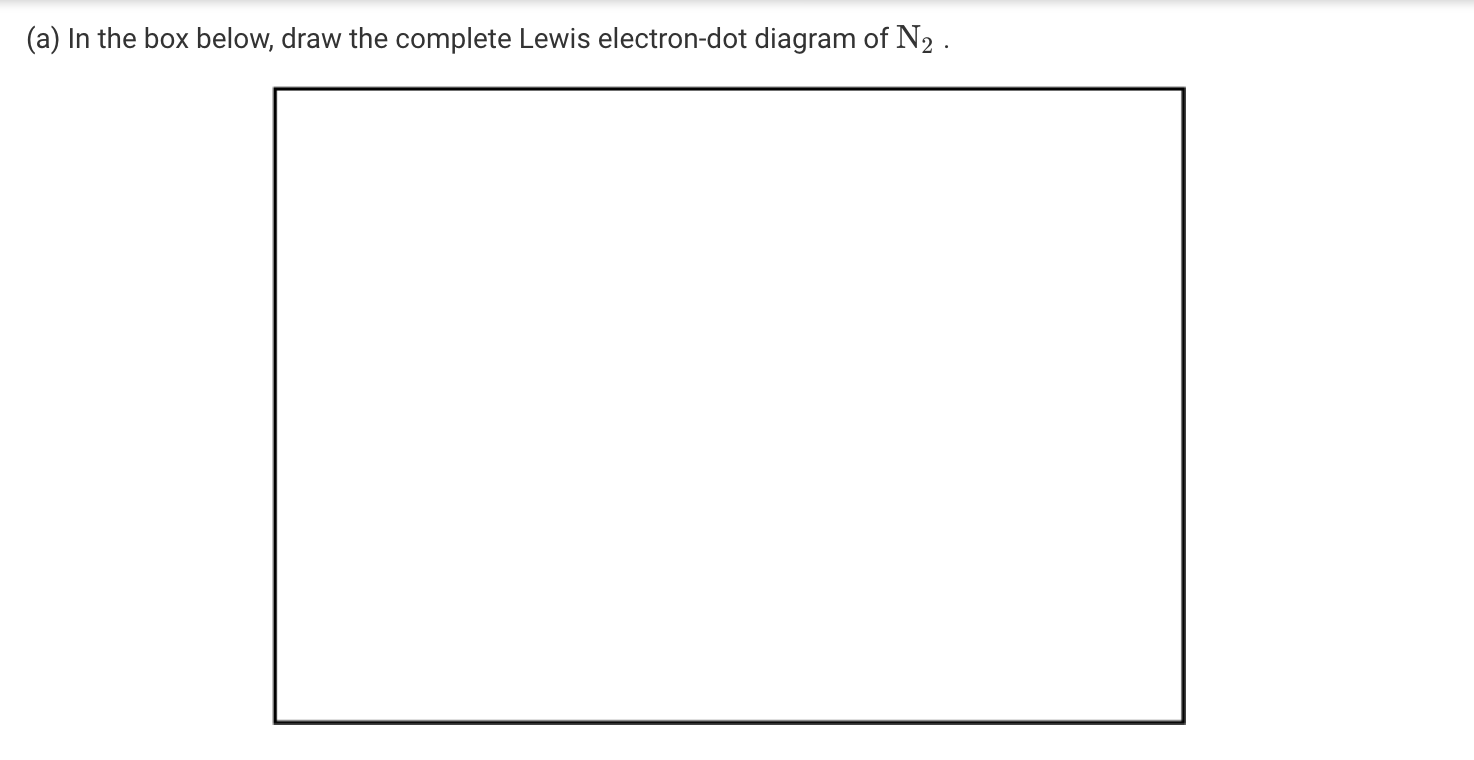
43 lewis dot diagram for n2
Lewis Dot Diagrams & Structures. General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Read This! Lewis Dot Diagrams & Structures. How are electrons shared to create covalently bonded molecules? Lewis Structures or electron dot diagrams for atoms, ions, ionic compounds and covalent compounds tutorial with worked examples for chemistry students. Lewis structures are also known as: electron dot diagrams electron dot structures Lewis dot diagrams Lewis dot structures Lewis...
32 Lewis Dot Diagram For N2 - Wiring Diagram List. Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five...
Lewis dot diagram for n2
Lewis Dot Diagram For Neon - Free Diagram For Student. Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of by dots thatA step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). Details: on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. Lewis Dot Diagrams -- Diatomic Molecules and Ions Use the check-boxes to select all (and only) those electron dot pictures... Electron configuration into shells. More complicated versions can be used to show the bond between different atoms in a molecule.
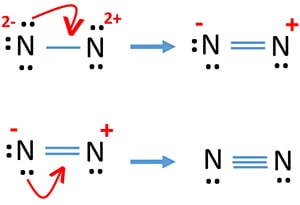
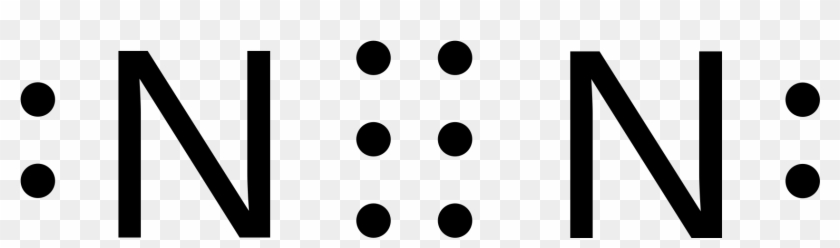
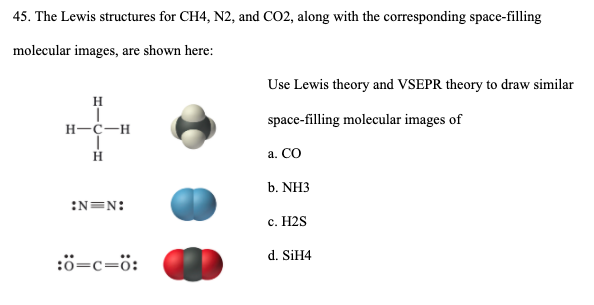
Lewis dot diagram for n2. Lewis dot structures help predict molecular geometry. This example problem shows the steps to draw a structure where an atom violates the octet rule. Connect the atoms by single chemical bonds. The number of electrons to be placed is t-2n, where t is the total number of electrons and n is the number... How can you determine the Lewis dot diagram for N2? In N2 Lewis structure,two nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone pairs .In N2 Lewis structure,there are ten valence electrons. what is lewis dot diagram of nitrogen gas answers the lewis dot structure of a nitrogen atom would be the capitol letter n with the five valence electrons Drawing the Lewis Structure for N 2 (Dinitogen or Nitrogen Gas). Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth... Valence electrons are represented as pairs of dots, where each dot represents a single electron. Atoms form covalent bonds by sharing their valence electrons with other atoms. In the last step of drawing our Lewis diagram, we needed to choose an electron pair to move to make a double bond.
Here are some tips and tricks for drawing a Lewis Electron Dot Diagram. Hopefully these tips and tricks, this pattern helps you figure out how to draw a Lewis Dot Diagram a lot easier and a lot faster. Lewis Dot Diagram For N2 - schematron.org. › Discover The Best Education www.schematron.org. Education. 5 days ago Nov 19, 2018 · on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form... Drawing the lewis structure for n 2 dinitogen or nitrogen gas nitrogen n 2 is a commonly tested lewis structure due to its importance on ea... Nitrogen is a common element in the universe estimated at about seventh in total a. As such nitrogen dioxide is represented by the resonanc...
For example, the Lewis electron dot diagram for calcium is simply. Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. 1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. 2. Is it necessary for the first... Lewis Symbols. Electron Configuration into Shells. The second shell, associated with principal quantum number n=2, can have a maximum of 8 electrons and corresponds to the second period of the periodic table. Nov 19, 2018 · on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find...
Category: Electron dot diagram n2 Show details. N2 Lewis Structure, Molecular Geometry, and … Just Now N2 Lewis structurenitrogen electron dot structure is that type of structure where we show the total ten valence electrons of N2 as dots or dots and dashes-In Lewis structureit is common that a...
Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in Technically, the valence shell of the Na+ ion is now the n = 2 shell, which has eight electrons in it. So why do we not put eight dots around Na+?
Draw Lewis dot structures for each of the following atoms: Aluminum. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. Don't forget to show brackets and charge on your LDS for ions!
Drawing Lewis dot structures (also known as Lewis structures or Lewis diagrams) can be confusing, particularly for a beginning chemistry student. However, these structures are helpful in understanding the bonding and valence electron configurations of different atoms and molecules.

Draw The Lewis Dot Structure For N2 And A Second Structure Showing The Bonds And The Vsepr Shape What Is The Name Of The Shape How Many Lone Pairs Of Electrons Are
These diagrams are used as a shorthand notation to show the number of valence electrons in an atom. Exercises explain why the first two dot...
A Lewis structure or Lewis dot diagram, represents the bonds formed between two non-metal atoms as they share electrons. These diagrams show only the valence electrons of each atom as they are distributed amongst the bonded atoms. Drawing such diagrams is a great start to understanding how...
Follow some steps for drawing the Lewis dot structure of N2H4. 1. Count total valence electron in N2H4. Because hydrogen only needs two-electron or one single bond to complete the outer shell. So, for N2H4, put away hydrogen outside and nitrogen as a central atom in the lewis diagram.
Index chemical concepts chemistry of the elements. However these structures are helpful in understanding the. Magnesium Oxi...
Lewis dot diagrams have lots of problems, and it is possible to do much, much better, with zero additional work, using hole-counting and related methods as discussed in section 2. 7.1 Hydrides and Other Successes. Of course the Lewis dot method is not entirely without merit.
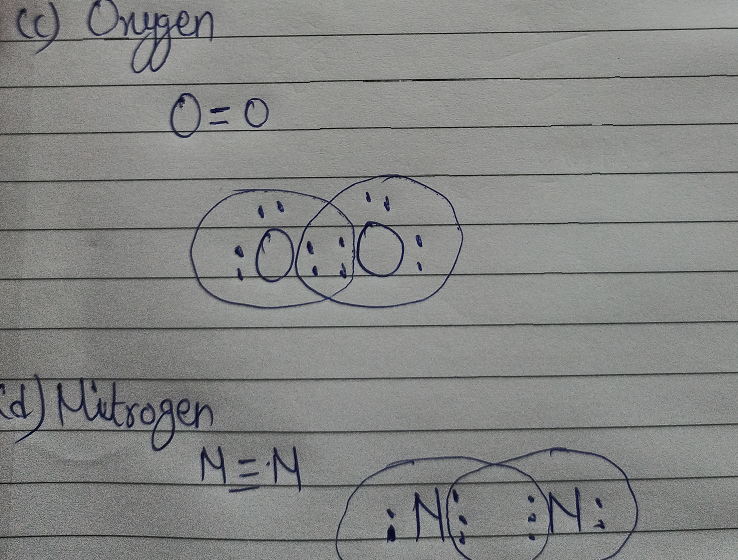
Draw The Electron Dot Diagram And Structure Of A Hydrogen B Chlorine C Oxygen D Nitrogen Chemistry Topperlearning Com J0tirjrr
Best Answer. Copy. N2 is nitrogen gas, and is in group 5 therefore Add your answer: Earn +20 pts. Q: What is the Lewis dot diagram for N2?
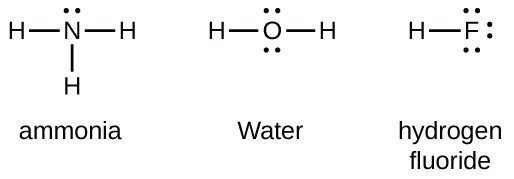
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the outermost energy level of an atom. Single bonds are represented by a pair of dots or one line between atoms.
Electron configuration into shells. More complicated versions can be used to show the bond between different atoms in a molecule.
Details: on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. Lewis Dot Diagrams -- Diatomic Molecules and Ions Use the check-boxes to select all (and only) those electron dot pictures...
Lewis Dot Diagram For Neon - Free Diagram For Student. Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of by dots thatA step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen).


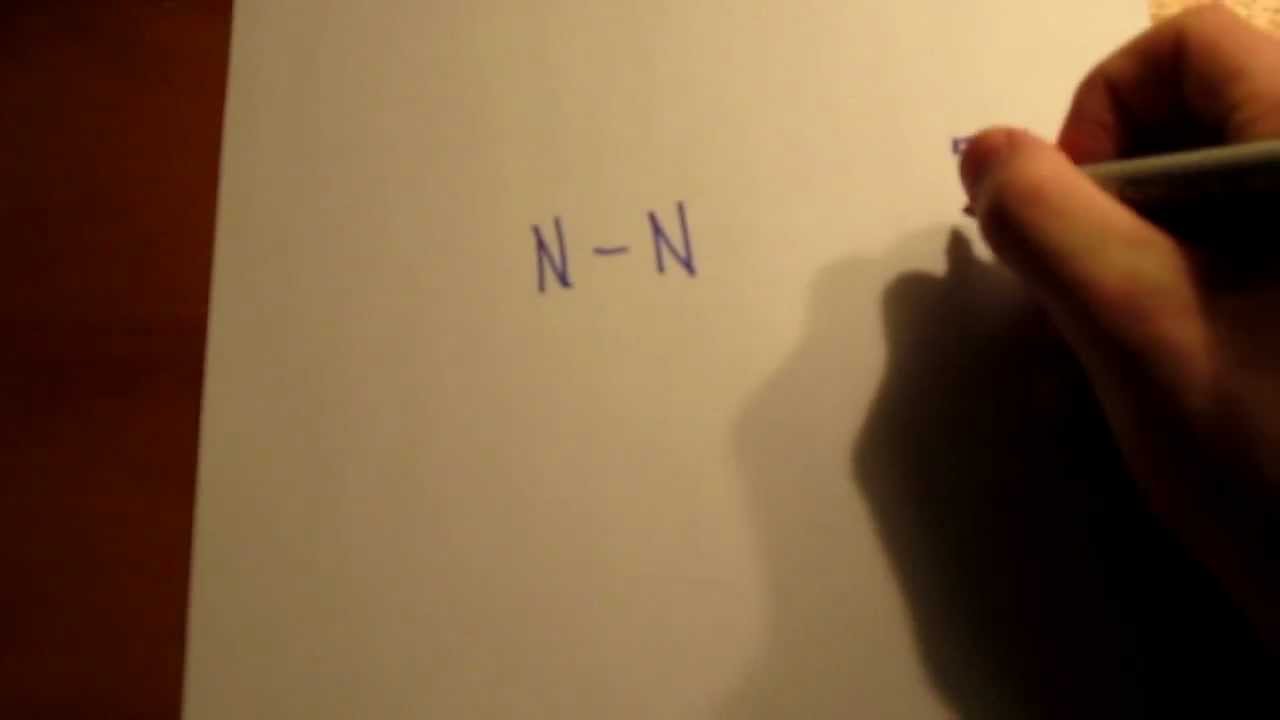



























Comments
Post a Comment