42 orbital diagram for silver
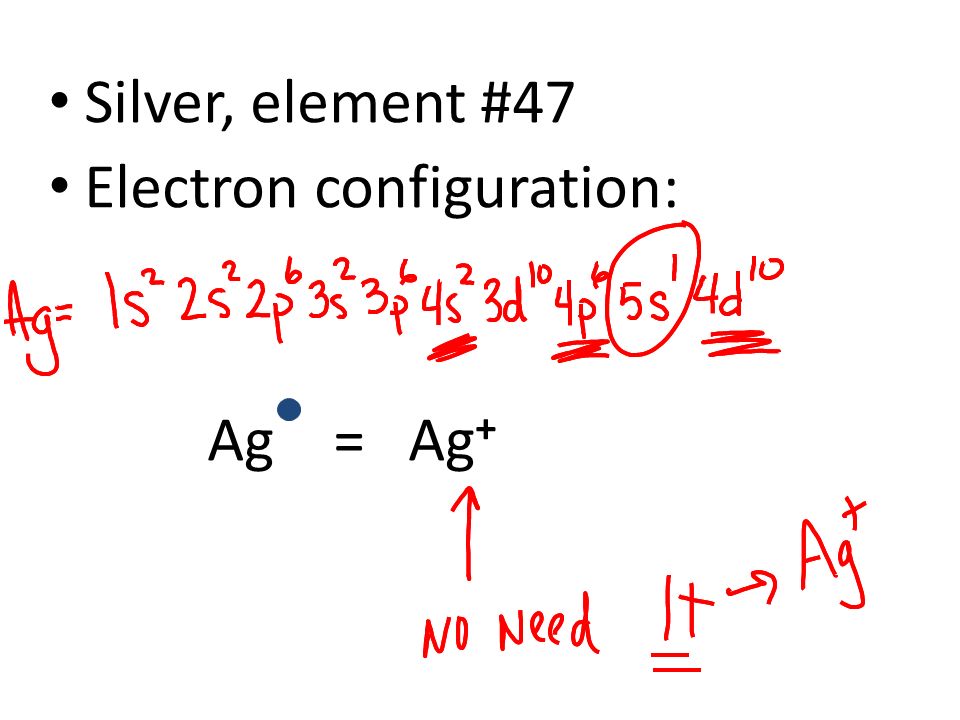
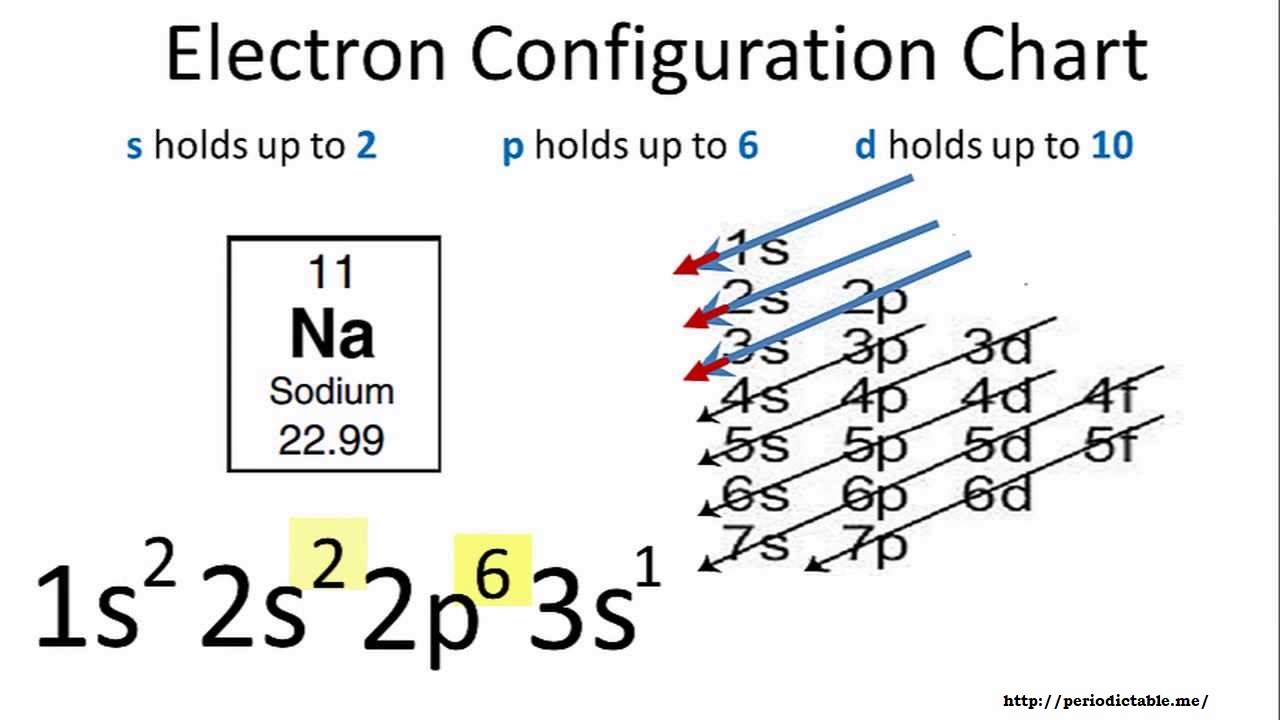
Well, we can write the electronic configuration of the silver atom... A quick glance at the Periodic Table tells us that Z_"Ag"=47..and so for the NEUTRAL atom.... We use the Aufbau process to write the electronic distribution of the SILVER atom... underbrace(1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)3d^(10)4s^(2)4p^(6))_"electronic configuration of krypton"5s^(1)4d^(10) That the configuration shows 5s^(1 ... The atomic structure of the atom depends on the electronic configuration in which the electrons are arranged inside the atoms. This electronic configuration is ...1 answer · Top answer: Hint: According to Dalton’s theory the atom is the most basic form of matter but the atom contains various subatomic particles like the electrons and ...
The silver atom used in the experiment has a total of 47 electrons, 23 of one spin type, and 24 of the opposite. Because electrons of the same spin cancel each other out, the one unpaired electron in the atom will determine the spin. ... An effective visual on how to assign spin directions can be represented by the orbital diagram (shown ...
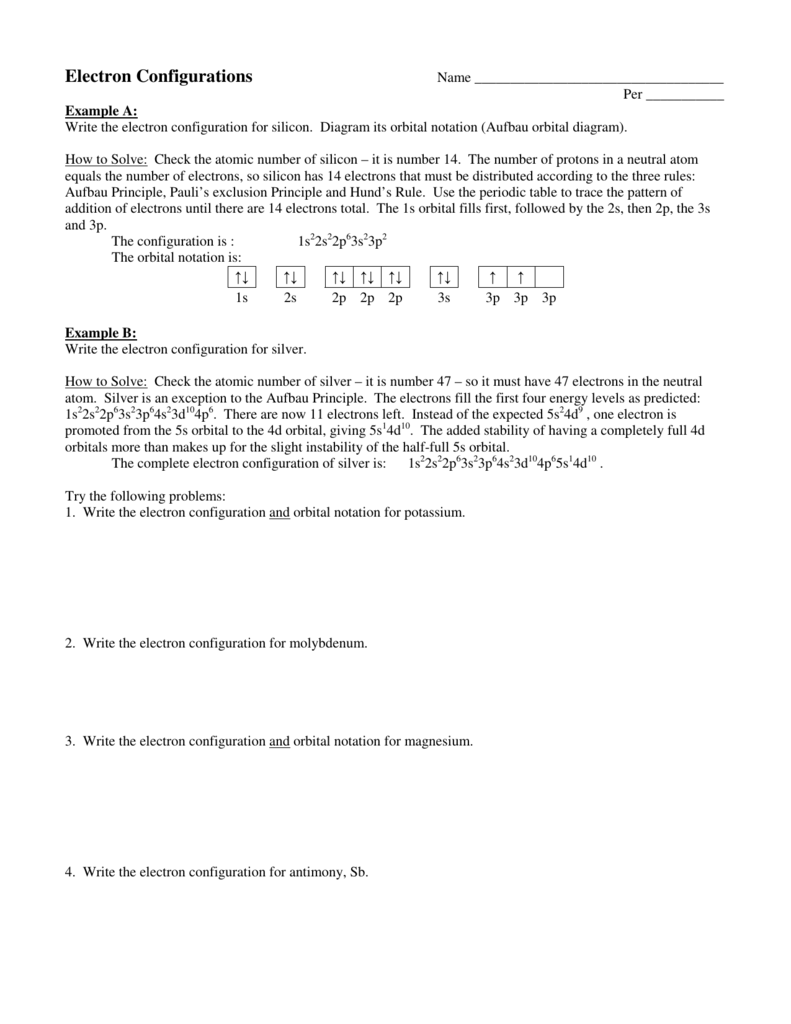
Orbital diagram for silver
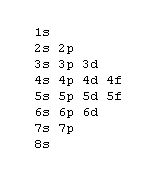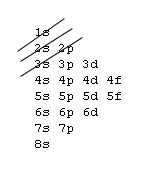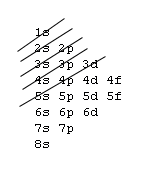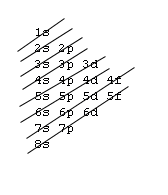
Silver-Line SL-1218R Orbital Floor Refinisher Owners Manual Thank you for purchasing a SL-1218R floor refinisher. When used properly this unit will perform well as a rental machine. Please review and follow the suggested procedures for operation of this machine. Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ... 5, Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Sb, Te, I, Xe ... The electron configuration of an element is a list of the atomic orbitals which ...
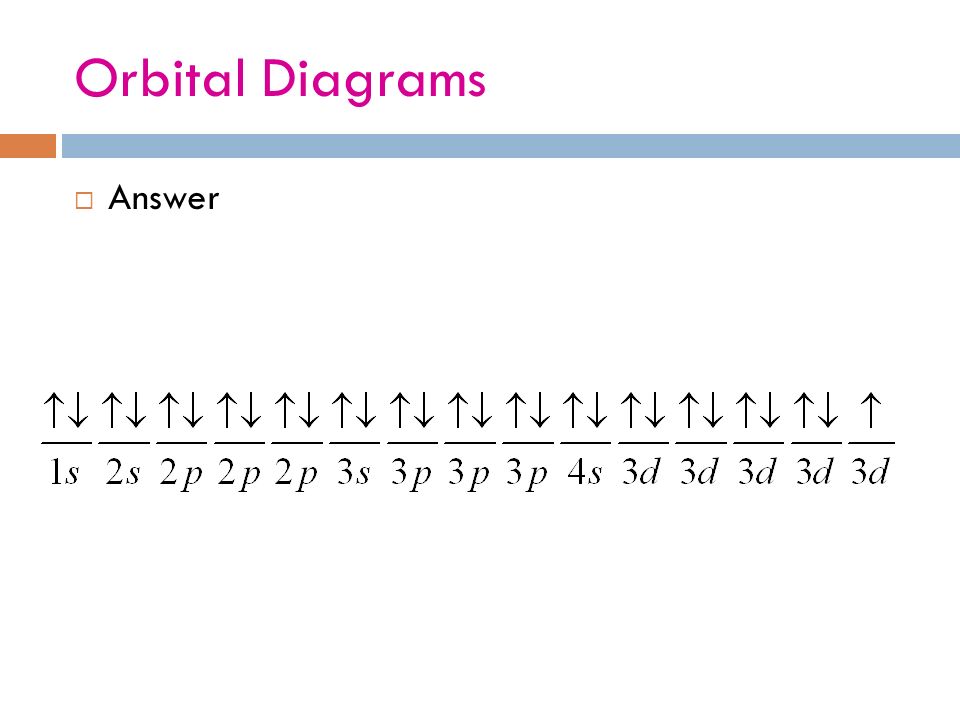
Orbital diagram for silver. Silver is a relatively soft, shiny metal. It tarnishes slowly in air as sulfur compounds react with the surface forming black silver sulfide. Uses. Sterling silver contains 92.5% silver. The rest is copper or some other metal. It is used for jewellery and silver tableware, where appearance is important. Silver (Ag) has an electron configuration of [Kr] 4d 10 5s 1. The element is much more stable and has a lower energy when the 4d orbital is filled, so one electron is placed there, rather than in the 5s orbital. When it is ionized, the electron is removed from the outermost shell, which is the 5s orbital. So the electron configuration for Ag ... Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. 6. Draw the orbital energy (i.e., box) diagram for the electrons beyond the [Kr) core of the element silver (Ag). If all the electrons are paired in the orbital energy diagram, the element is said to be diamagnetic, meaning that atoms of the element will interact weakly with an external magnetic field.
Silver (Ag). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of silver-108 (atomic number: 47), an isotope of this element. The nucleus consists of 47 protons (red) and 61 neutrons (orange). 47 electrons (white) successively occupy available electron shells (rings). Orbital box notation uses boxes or horizontal lines to represent orbitals and arrows to represent electrons. Thus, there are two 1s electrons, two 2s electrons, and six 2p electrons. Write the electron configuration for silver. Sr +2 8. Orbital notation for silver using noble gas abbreviation? Electron configuration is how the electrons are distributed among the various atomic orbitals in an atom. Only two electron can be located in each orbital. The "s" sublevel contains 1 orbital, the ... Ag, or silver, has the atomic number 47. Its orbital notation is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10.
Answer: Silver (Ag) has an electron configuration of [Kr] 4d 10 5s 1. The element is much more stable and has a lower energy when the 4d orbital is filled, so one electron is placed there, rather than in the 5s orbital. When it is ionized, the electron is removed from the outermost shell, which is the 5s orbital. Orbital diagram of Silver (Ag) 48: Orbital diagram of Cadmium (Cd) 49: Orbital diagram of Indium (In) 50: Orbital diagram of Tin (Sn) 51: Orbital diagram of Antimony (Sb) 52: Orbital diagram of Tellurium (Te) 53: Orbital diagram of Iodine (I) 54: Orbital diagram of Xenon (Xe) 55: Orbital diagram of Caesium (Cs) 56: Orbital diagram of Barium (Ba ... Melting Point of Silver: 961.93 °C This is similar to the electron configuration, but it includes a diagram of the Aufbau Principle: Fill lower energy sublevels before higher energy sublevels. The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. An orbital is a region of space that an electron can exist in. For the diagram you start with the 1 s orbital and then 2s, 2p, and so on. Each orbital can hold 2 electrons and each arrow ...
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Silver (Ag) has an atomic mass of 47. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
2. Electron Configuration (quicker to draw than orbital filling diagrams) 2 2 Ex. O 2 1s 2s 2p4 3. Electron Dot shows only the valence (outer energy level) electrons . . Ex. :Oxygen atom . O . 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram
The orbital diagram for hydrogen can be represented in the following way. This notation uses a box to represent the orbital, the label for the orbital and an arrow to represent the electron. The electronic configuration for hydrogen can be written as 1s 1. This is a short-hand notation which identifies the level, the sublevel and the number of ...
Dec 5, 2020 — Silver Electron Configuration (Ag) with Orbital Diagram ... Electron Configuration For Silver: Silver is a chemical element that has a chemical ...
Ag (Silver) is an element with position number 47 in the periodic table. Located in the V period. Melting point: 961.9 ℃. Density: 10.49 g/cm 3 . The order of filling the orbitals with electrons in the Ag atom is an exception to the rule. Expected electronic configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9.
2. Electron Configuration (quicker to draw than orbital filling diagrams) Ex. O2 1s2 2s2 2p4. 3. Electron Dot shows only the valence (outer energy level) electrons. . Ex. Oxygen atom . O :. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams . for the following elements. Table: Element Orbital Filling Diagram
Silver. Electronic configuration. Electronic configuration. 1s22s22p63s23p63d104s24p64d105s1. >> Back to key information about the element ...
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
Download scientific diagram | 2 Orbital diagram. ... Silver, Chemistry and Catalysis | ResearchGate, the professional network for scientists.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
7. (8 pts.) Construct an electron configuration and orbital diagram for the element silver (Ag). For the electron configuration you may use the noble gas shorthand representation if you wish. Question: 7. (8 pts.) Construct an electron configuration and orbital diagram for the element silver (Ag).
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
1 answerTo create the orbital configuration, we can refer to the orbital diagram below. As silver has an atomic number of 47, a neutral atom has 47 protons...
Silver has electronic configuration as [Kr] 4d 10 5s 1. The s orbital ( 5s) has only one electron.And the s orbital here has n = 5 (as it is in the fifth period) and l = 0 . The s orbital is symmetric in space (sphere) which has only one orientation and hence has no "different" orientations each of which is represented by a particular value of m l.So m l can take only one value and that is 0.

Electron Configurations And Orbital Diagrams Maximum Number Of Electrons In Each Sublevel Maximum Number Of Electrons In Each Sublevel Maximum Number Ppt Download
5, Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Sb, Te, I, Xe ... The electron configuration of an element is a list of the atomic orbitals which ...

Describe The Orbital Notation And Electron Configuration Notation To Describe The Placement Of All Of Brainly Com
Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ...
Silver-Line SL-1218R Orbital Floor Refinisher Owners Manual Thank you for purchasing a SL-1218R floor refinisher. When used properly this unit will perform well as a rental machine. Please review and follow the suggested procedures for operation of this machine.
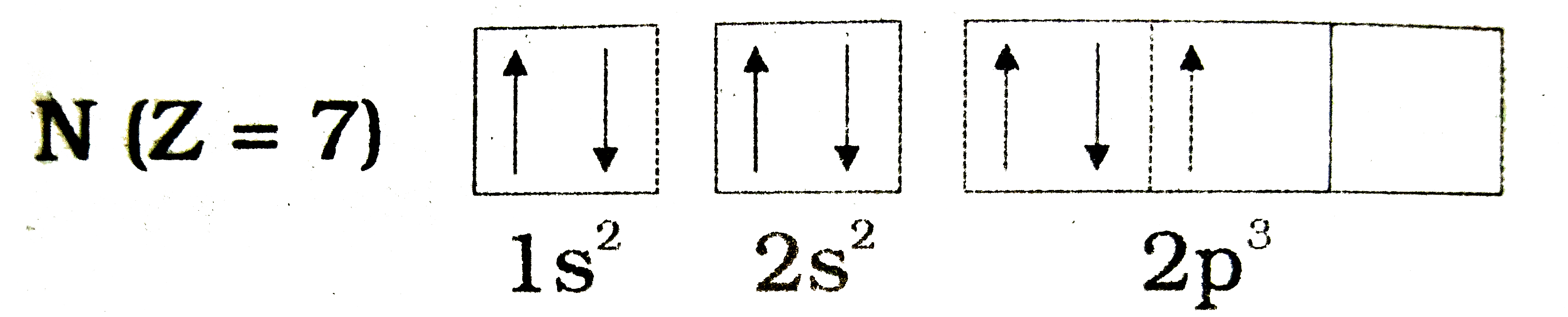
Following Orbital Diagram Shows The Electronic Configuration Of Nitrogen Atom Which Rule Does Not Support This Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images Brs Qb Phy Sci X C06 E01 017 Q01 Png Width 80

1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2

What Element Is Represented By The Orbital Diagram Above A Iron B Calcium C Krypton D Zinc Brainly Com







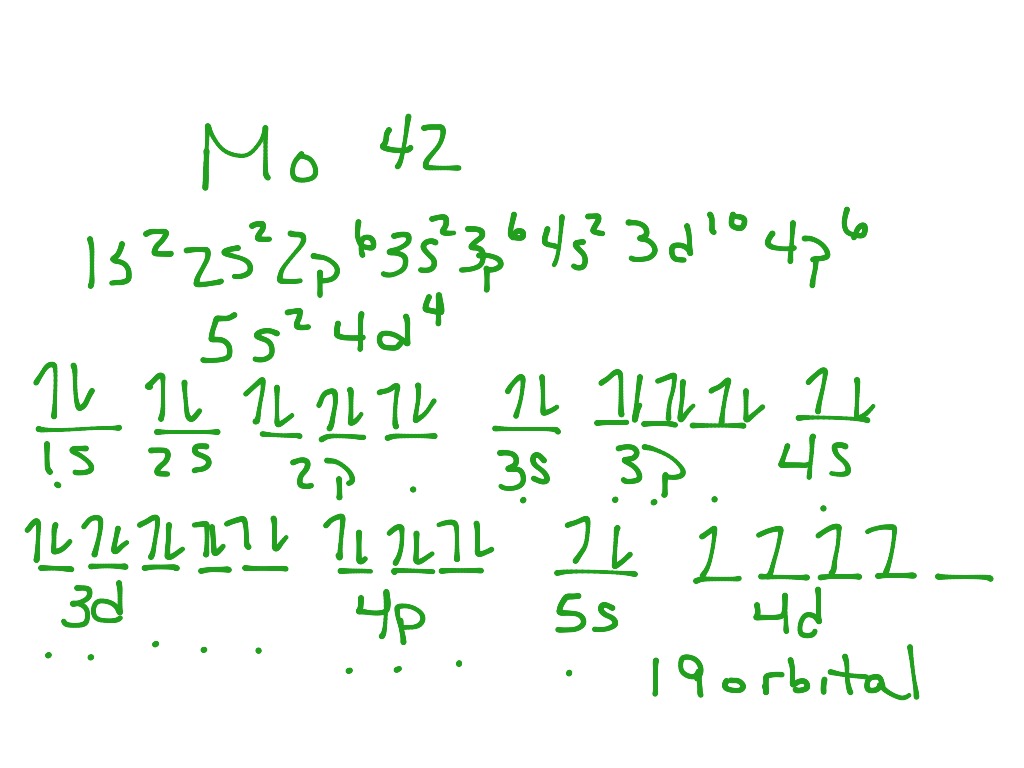





:max_bytes(150000):strip_icc()/Silver-58b601c15f9b5860464c0e8f.jpg)










Comments
Post a Comment