30 Oct 2015 — In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group ...1 answer · http://mrdesjardinsdd12wiki.wikispaces.com/Nomenclature-+Chapters+7,+8,+9 Explanation: In order to come up with this answer, you first need to know the ... Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence ...
Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple covalent bonds. The ...2 answers · 0 votes: In exactly the same way as you’d do it for any simple, covalently bonded compound. First ...
Lewis dot diagram of n2
We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each ...17 Oct 2013 · Uploaded by Wayne Breslyn
Lewis dot diagram of n2. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each ...17 Oct 2013 · Uploaded by Wayne Breslyn

Solved Lewis Dot Diagrams Diatomic Molecules And Ions Use Chegg Com

1

N2 Lewis Structure Molecular Geometry And Hybridization Techiescientist

N2 Lewis Structure Lewis Structure N2 Hnd Assignment

What Is The Lewis Structure For N2 Nitrogen Gas Quora

1
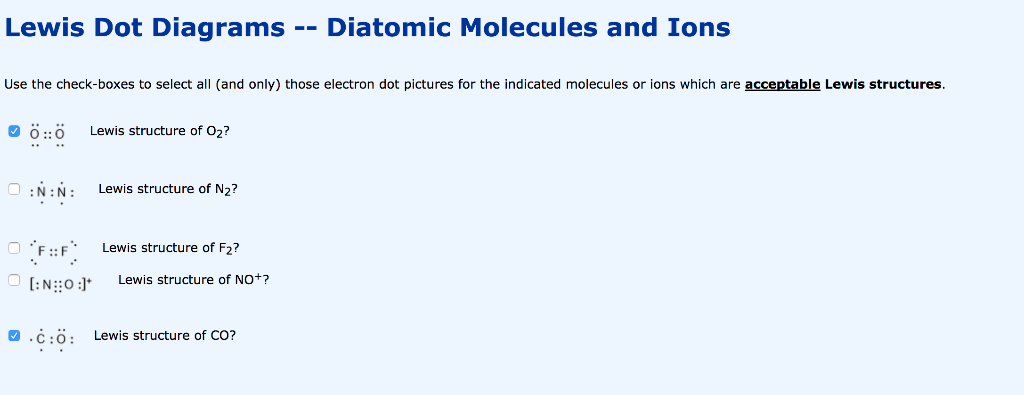
No2 Nitrogen Dioxide Lewis Dot Structure Science Trends

No2 Nitrogen Dioxide Lewis Dot Structure Science Trends

How To Draw The Lewis Structure For N2 Drawing Easy

Solved 4 Which Description Of The Bond Between Nitrogen Atoms In N2 Is Correct Course Hero
Lewis Structures And Covalent Bonding

N2o Lewis Structure Nitrous Oxide Laughing Gas What S Insight
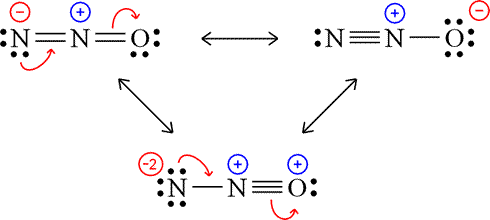
How Many Resonance Structures Can Be Drawn For N 2o Socratic

What Is The Lewis Structure Of N2 Socratic
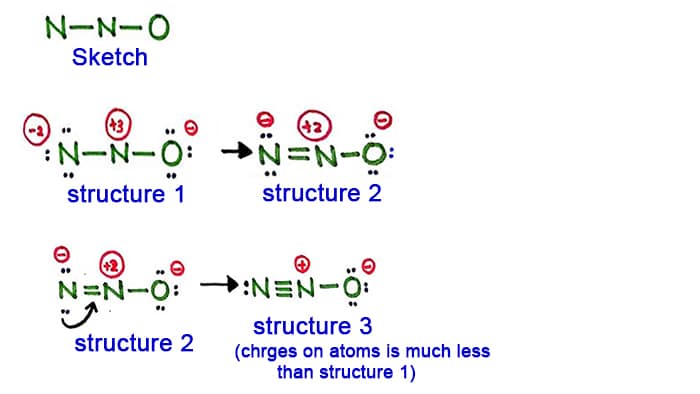
N2o Lewis Structure Resonance Structures Oxidation Number

How To Determine The Lewis Dot Diagram For N2 Quora
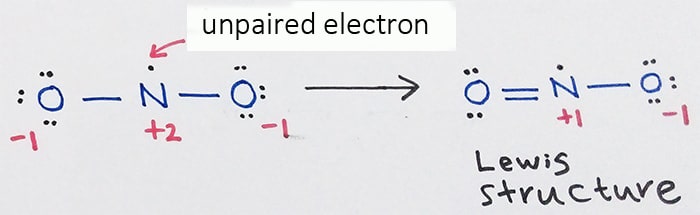
Lewis Structure For No2 Nitrogen Dioxide Oxidation Number

Lewis Structures Single Double Triple Bonds Video Lesson Transcript Study Com
Solved A In The Box Below Draw The Complete Lewis Chegg Com

Chem 101 Lewis Structures

No Lewis Dot Structure Science Trends

Lewis Structure Of N2 Nitrogen Gas Youtube

Lewis Dot Structure Practice Flashcards Quizlet

Solved 3 Shown Below Is The Lewis Structure For Nitrogen Chegg Com

Lewis Structure Wikipedia

N2o Lewis Structure Nitrous Oxide Laughing Gas What S Insight

Write Lewis Structure Of N2 Clutch Prep

Draw The Electron Dot Structure Of N2 Molecule Science Carbon And Its Compounds 11435175 Meritnation Com

Nitrogen N2 Pubchem

Covalent Bond And Lewis Dot Structure H2o Co2 Video Khan Academy

Multiple Covalent Bonds Chemistry For Non Majors
3 6 Writing Lewis Structures Chemistry Libretexts

N2 Lewis Structure Lewis Dot Structure For Nitrogen N2 Gas Of N2 Gas Youtube
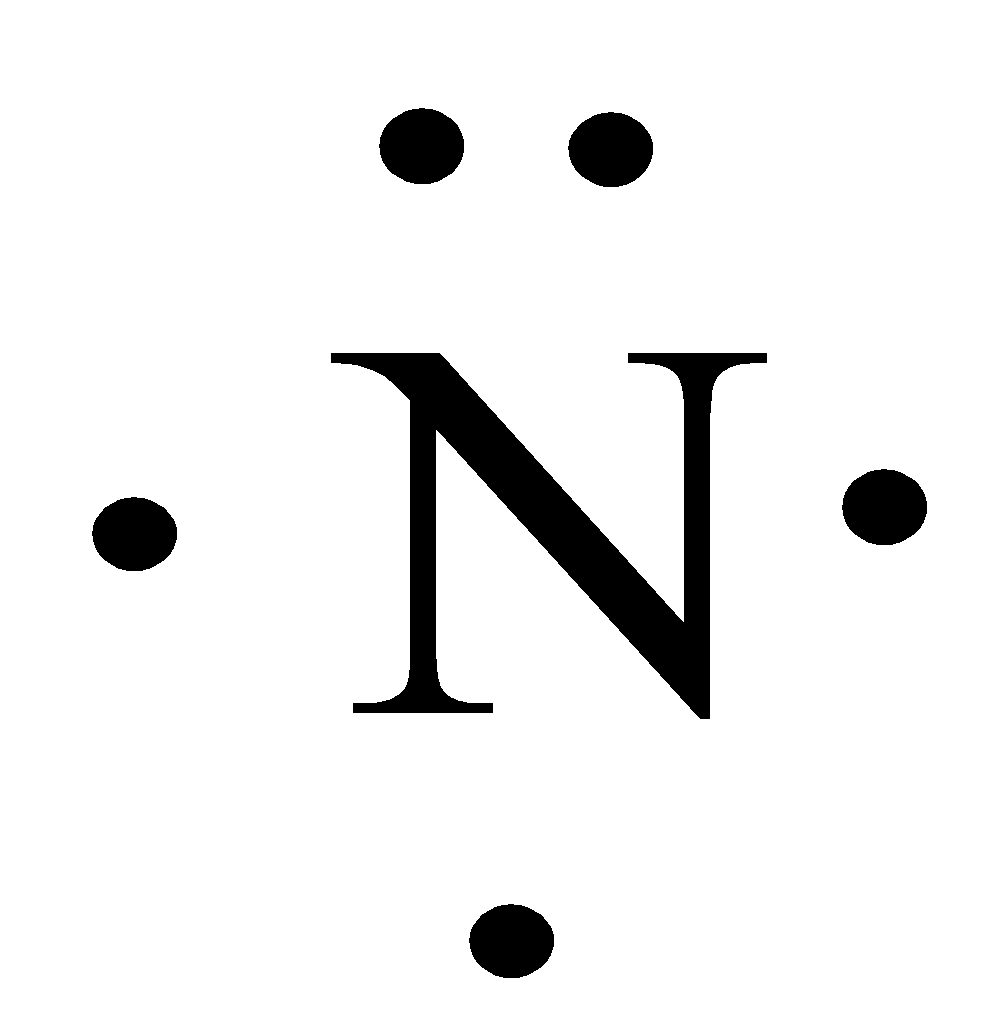
How Do You Writedraw The Lewis Structure For N Class 11 Chemistry Cbse
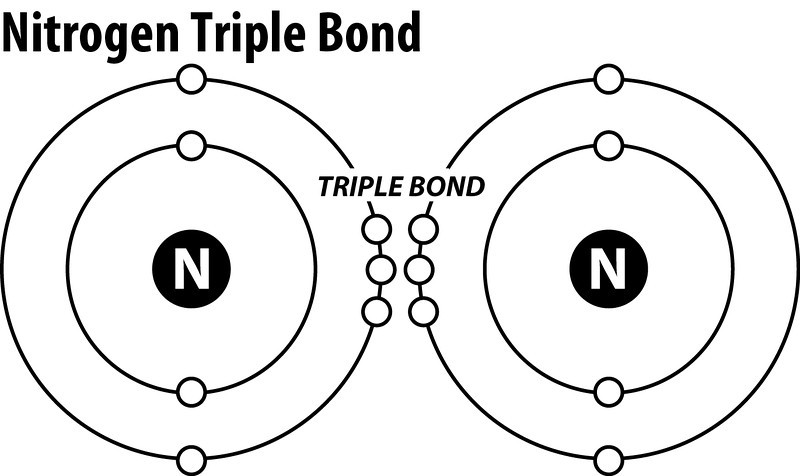
What Is The Lewis Structure Of N2 Socratic

Lewis Electron Dot Structures Chemistry For Non Majors
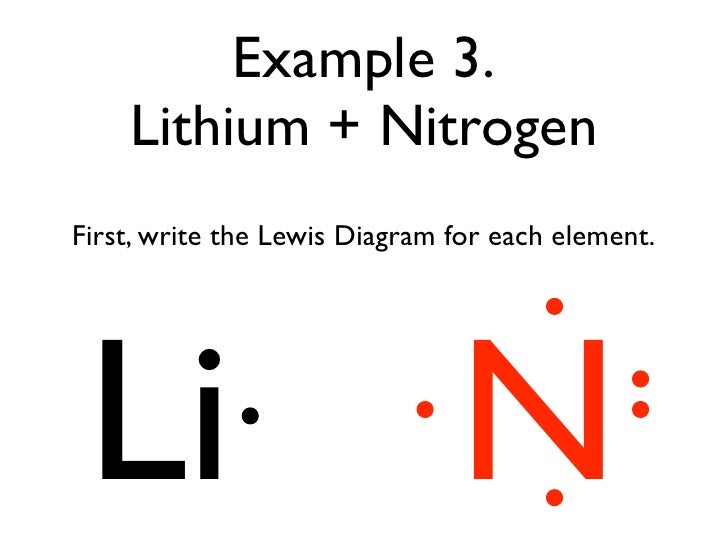
Bonding Basics

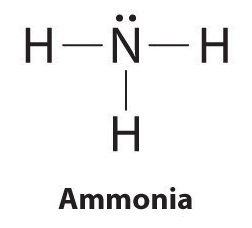
Write Electron Dot Structure For The Following A Nitrogen Atomsb Methane Brainly In



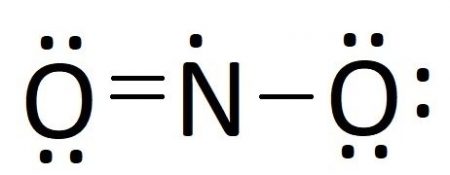





























Comments
Post a Comment